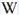Base de données des minéraux luminescents
BARYTE
Formule chimique: BaSO4
Famille: Sulfates
Statut: IMA-GP
Système cristallin : Orthorhombique
Minéral de vitrine: OUI
Noms associés (variétés luminescentes, noms discrédités, synonymes etc.): barytine, hokutolite, Lepastrum,
Luminescence:
Couleurs UV longs (365nm): |
Blanc jaunâtre (crème) , Blanc bleuté , Jaune pâle , Jaune-orange , Orange , Blanc verdâtre , Jaunâtre , | ||
Intensité OL:Moyenne | Fréquence OL:Souvent | ||
Couleurs UV moyens (320nm): |
Blanc jaunâtre (crème) , Blanc bleuté , Jaune pâle , Jaune-orange , Orange , Blanc verdâtre , Jaunâtre , | ||
Intensité OM:Moyenne | |||
Couleurs UV courts (254nm): |
Blanc jaunâtre (crème) , Blanc bleuté , Jaune pâle , Jaune-orange , Orange , Rose saumon (rose orange) , Blanc verdâtre , Jaunâtre , | ||
Intensité OC:Moyenne | Fréquence OC:Rarement | ||
Photo Ondes Courtes (254nm)

Baryte (fluo cream) in calcite (fluo red) SW,
Franklin Mine, Sussex Co., New Jersey, USA
Col. G. Barmarin; Photo: G. Barmarin
Vous avez une photo de ce minéral que vous voudriez voir figurer dans la galerie? Contactez-nous!
Phosphorescence (au sens commun du terme) observable à l'oeil nu:
Type d'UV |
Couleur |
Intensité |
Fréquence d'observation |
|---|---|---|---|
UV longs (365nm): | Blanc jaunâtre (crème) | UV moyens (320 nm): | Blanc jaunâtre (crème) | UV courts (254 nm): | Blanc jaunâtre (crème) |
Commentaires:
La barytine est l'un des premiers matériaux luminescents à partir duquel la célèbre pierre de Bologne a été obtenue. Néanmoins, à ce jour, la compréhension de la luminescence naturelle de la barytine est très parcimonieuse. On sait depuis longtemps que certains spécimens de barytine sont fluorescents sous UV et émettent une lumière blanche, jaune, verte ou orange. Dans les spectres de luminescence en régime permanent de la barytine, différentes bandes de luminescence ont été détectées, de l'UV à la partie rouge du spectre. Cependant, seuls les centres de luminescence UO22+ et Eu2+ ont été identifiés avec certitude (Tarashchan 1978 ; Gaft et al. 1985).
Activateur(s) et spectre(s):
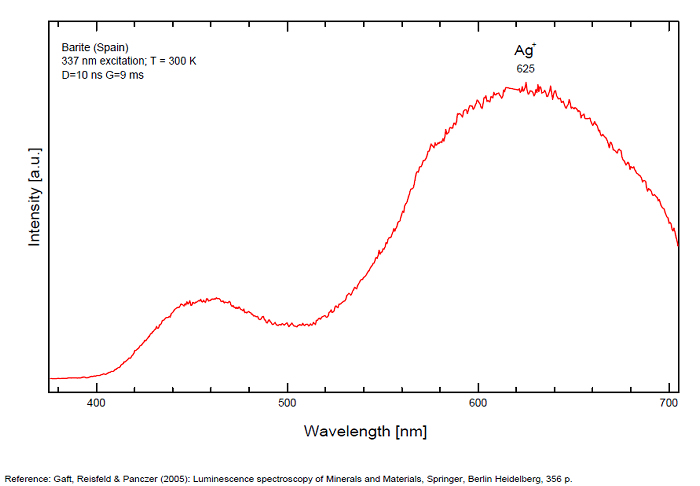
Activateur(s): (UO2)2+ (ion Uranyle) en impureté, Pb2+, S2-, ST (Singlet-triplet)-Matière organique en impureté, Cu+, Eu2+, Ce3+, Tb3+, Ag+, Nd3+, Bi2+, Bi3+,
Pics dans le spectre (nm):
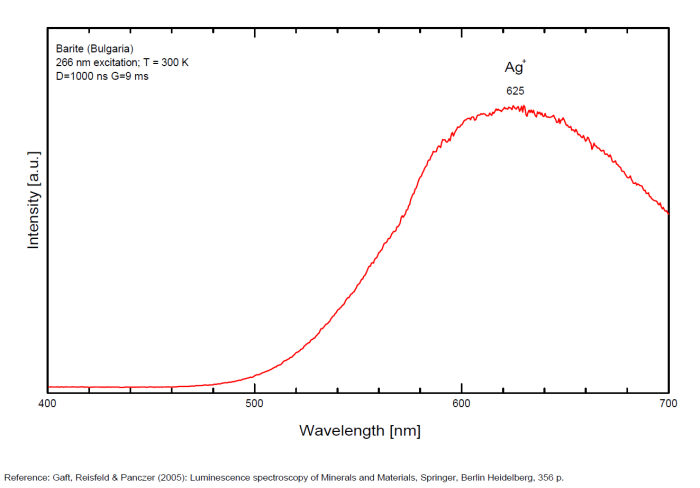
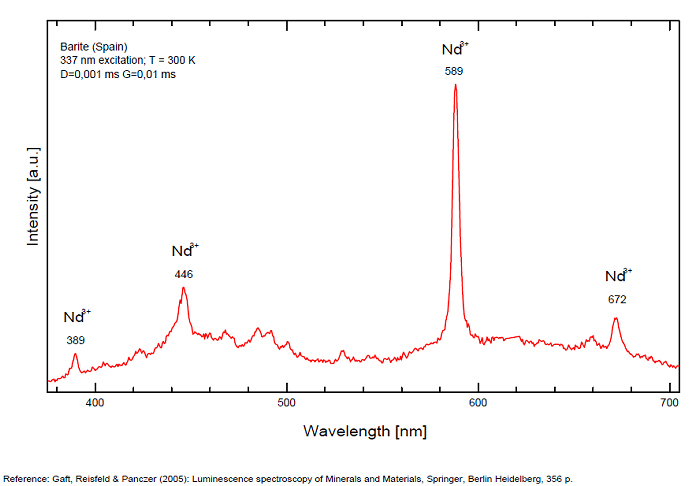
Bi3+ : 426-430nm band 80nm half-width ( τ = 750 microsecondes) Bi2+ replacing Ba2+ : narrow band 625nm ( τ = 5 microsecondes) Ag+ : very broad band (150 nm half-width) peaking at 635nm ( τ = 225 microsecondes) Ce3+ : 302, 305, 330, 360nm Nd3+ : 389, 446, 589, 672nm Eu3+ : 592, 620, 701nm Tb3+ : 488, 544nm UO22+ : 501, 522, 544, 568 Eu2+ : 375, 613, 615, 697nm Pb : blue part of the spectrum
Spectrum: Michael Gaft, Petah Tikva, Israel. Plot: Institute of Mineralogy, University of Vienna, Austria, with permission of the authors.
Galerie de spectres:
 ...
...  Accès à la galerie complète (3 images au total)
Accès à la galerie complète (3 images au total)
Commentaires sur les spectres et les activateurs (*):
It is known that the luminescent centers Eu2+ (1.24–1.40 Å), Ce3+ (0.88–1.02 Å), Nd3+ (0.99–1.15 Å), Tb3+ (0.89–1.09 Å), Ag+ (1.13–1.26 Å), Sn2+ (0.93 Å) substitute for Ba2+ (1.35–1.44 Å) or Sr2+ (1.10–1.27 Å), which are in 12-fold coordination in the barite structure (all ionic radii are for the 6-coordination form) (GOROBETS, 2002; GAFT et al., 2005); A possible accommodation for the established in this study Eu3+ (0.97–1.13 Å) is also isomorphic substitution for Ba2+ or Sr2+ Activators: (UO2)2+ UO22+ can be determined from the vibrational structure and the long decay time of the luminescence band. Two different types of uranyl could be detected in Baryte: thin films of uranyl mineral considered to be a result of chemical adsorption (GOROBETS, 2002) and a solid solution of uranyl ion in barite crystal. Activators: Eu2+ Eu2+ can be determined from the spectral position, the half-width and the characteristic decay time of the luminescence band. Mn2+ and Ag+ can be determined by comparing luminescence bands spectral parameters to those of synthesized BaSO4−Mn and BaSO4−Ag. The luminescence spectrum (excitation of 266 nm, without delay, broad gate of 9 ms) of the studied endogenous barite contains 2 relatively narrow ultraviolet bands: one peaking at 306 nm and the other at 375 nm. The first band has a very short decay time and disappears after D = 50–100 ns. Such a combination of spectrum and decay time parameters is evidence that the luminescence is connected with Ce3+. The emission of Ce3+ corresponds to transitions between 5d1 and 4f1 electronic configurations. The second band has a longer decay time of approximately 1 μs and belongs to Eu2+ (GAFT et al., 2005). The Emission spectra of Eu2+ result from electronic transitions between 4f7 and 4f65d1 electronic configurations. After a delay of several μs, the Eu2+ emission becomes much weaker and very weak narrow lines appear, peaking at 488, 544 and 615 nm ). These lines are connected with trivalent rare-earth elements, which are characterized by relatively long decay times of hundreds of μs: the first 2 lines certainly belong to Tb3+ and the last one is principally typical for Eu3+ (GAFT et al., 2005). While Eu2+ luminescence is common, Eu3+ emission is here detected for the first time in barite. Under the excitation of 532 nm, a luminescence line at 615 nm dominates the spectrum, accompanied by several lines near 700 nm. Such behavior confirms that Eu3+ is responsible for these luminescence lines. The emission of Eu3+ corresponds to f–f transitions – from the excited 5D0 level to the 7Fj (j=0, 1, 2, 3, 4, 5, 6) levels of the 4f6 configuration. (Spectroscopic study of barite from the Kremikovtsi deposit (Bulgaria) with implication for its origin, MAYA DIMOVA, GERARD PANCZER & MICHAEL GAFT, ANNALES GÉOLOGIQUES DE LA PÉNINSULE BALKANIQUE, Vol67, 2006 See link below)
Ag+ (large pic 635 nm), Bi2+ in Ba2+ site (narrow band 625 nm), Bi3+ (426 nm), Eu2+ (375), Ce3+ (302, 330, 360nm), Nd3+ (446, 589nm) (Gaft)
Fe3+ or Mn4+ can be determined from the spectral-kinetic parameters of the luminescence bands.( After M. Gaft and I. Rudenkova, Laser-induced luminescence of barite after thermal treatment, Journal of Thermal Analysis and Calorimetry vol42, 1994)
In natural barite the narrow orange band with λmax=625 nm, Δ=40 nm and τ=5 μs is connected with Bi2+ center.
The narrow violet band with λmax=625 nm, Δ=35 nm and τ=1.7 ms is connected with Bi3+ center.
The broad red band with λmax=635 nm, Δ=140 nm and τ=270 μs is connected with Ag+ center. The emission bands of Ag+ result from d9s–d10 transitions
The broad red band with λmax=750 nm, Δ=110 nm and τ=350 μs is connected with Cu+ center.(The luminescence of Bi, Ag and Cu in natural and synthetic barite , M. Gaft, R. Reisfeldb, G. Panczer, G. Boulonc, T. Saraidarovb and S. Erlishd, Optical Materials, vol16, 2001)
The different emission spectra at excitations at 266 and 532 nm suggest at least 2 structural positions for Eu3+ in the barite crystal lattice.
(*)Les commentaires sur les spectres sont uniquement rédigés en Anglais
Meilleure(s) localité(s) pour la fluorescence (*):
- Franklin Mine, Franklin, New Jersey, USA (Baryte fluo yellow in massive calcite fluo red SW);
- 700 Level, Sterling Hill Mine, Ogdensburg, New Jersey, USA (Baryte fluo yellow in massive calcite fluo red SW);
- Edison Tunnel, Sterling Hill Mine, Ogdensburg, New Jersey, USA (Baryte fl. yellow LW and sphalerite fl.orange LW within willemite fl. Green SW, franklinite and massive calcite fl. red SW);
- Langban, Filipstad, Varmland, Sweden (fluo blue due to 1% Pb);
- Moscona Mine, Solís, Corvera de Asturias, Asturias, Spain (yellowish white LW)
- Book Cliffs area, Grand Junction, Mesa Co., Colorado, USA (white LW due to oil hydrocarbons + phosphor.);
- Silver Cliff Mining District, Custer Co., Colorado, USA (orange SW+MW+LW)
- Cerro Warihuyn (Huarihuyn), Miraflores, Huamalíes Province, Huánuco Department, Peru;
- Chengkou Co., Chongqing, China;
(*)Les données ne sont pas exhaustives et sont limitées à quelques localités remarquables pour la fluorescence
Référence bibliographique pour la luminescence:
- The Henkel Glossary of Fluorescent Minerals, Dr. Gerhard Henkel, Published by the FMS, 1989 ,
- Fluorescence: Gems and Minerals Under Ultraviolet Light, Manuel Robbins, 1994, Geoscience Press, ISBN 0-945005-13-X ,
- Luminescence Spectroscopy of Minerals and Materials, M. Gaft, R. Reisfeld, G. Panczer, Springer Editor, ISBN: 10 3-540-21918-8 ,
- Luminescent Spectra of Minerals, Boris S. Gorobets and Alexandre A. Rogojine, Moscow, 2002 ,
- Luminescenza nel regno minerale, Guido Mazzoleni, fotografia Roberto Appiani, Libri Sandit, 2010, ISBN 978-88-95990-63-7 ,
Référence pour la luminescence sur internet:
- Spectroscopic study of barite from the Kremikovtsi deposit (Bulgaria) with implication for its origin, MAYA DIMOVA, GERARD PANCZER & MICHAEL GAFT, ANNALES GÉOLOGIQUES DE LA PÉNINSULE BALKANIQUE, Vol67, 2006
- Laser-induced luminescence of barite after thermal treatment, M. Gaft, I. Rudenkova, JOURNAL OF THERMAL ANALYSIS AND CALORIMETRY, Volume 42, Number 1 (1994), 187-195
- The luminescence of Bi, Ag and Cu in natural and synthetic barite BaSO4, M.Gaft, R. Reisfeld, G. Gaft & al, Optical Materials, Volume 16, issue 1-2, p279-290, february-March 2001
- Der Aufschluss, Vol.48, n°2 March/April 1997 by Fritz Blatter;
Images:
- Langban, baryte activated by 1% Pb: http://www.mindat.org/photo-433931.html
- Colorado USA (white LW due to oil hydrocarbons): http://www.mindat.org/photo-290468.html
- Chengkou Co., Chongqing, China: https://www.mindat.org/photo-955190.html & https://www.mindat.org/photo-955692.html
- Moscona Mine, Asturias, Spain: https://www.mindat.org/photo-507652.html
- Silver Cliff Mining District, Colorado, USA: https://www.mindat.org/photo-1031168.html
- Silver Cliff Mining District, Colorado, USA SW : https://www.mindat.org/photo-1030553.html
Référence minéralogique sur internet:
 http://www.mindat.org/show.php?name=Baryte
http://www.mindat.org/show.php?name=Baryte
 http://webmineral.com/data/Baryte.shtml
http://webmineral.com/data/Baryte.shtml
Recherche sur Internet:
 Recherche d'images sur 'Google Image'
Recherche d'images sur 'Google Image'
 Recherche de documents en français sur Google
Recherche de documents en français sur Google
 Recherche de documents en toutes
langues sur Google
Recherche de documents en toutes
langues sur Google
Une requête ne donnant pas de résultat signifie uniquement qu'aucune référence de ce type n'existe dans la base mais en aucun cas qu'elle n'existe pas dans l'absolu. Si vous considérez avoir trouvé une erreur ou une omission, merci de nous le signaler via la page contact en n'oubliant pas de citer la source de l'information.