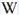Base de données des minéraux luminescents
BENITOITE
Formule chimique: BaTiSi3O9
Famille: Silicates
Statut: IMA-GP
Système cristallin : Hexagonal
Minéral de vitrine: OUI
Luminescence:
Couleurs UV longs (365nm): |
Rouge , | ||
Intensité OL:Faible | Fréquence OL:Rarement | ||
Couleurs UV courts (254nm): |
Blanc bleuté , | ||
Intensité OC:Très forte | Fréquence OC:Toujours | ||
Photo en lumière du jour
Photo Ondes longues (365nm)

BENITOITE, UVLW ;
Photo and Copyright: G. Barmarin
Photo Ondes Courtes (254nm)

BENITOITE;
UVSW
Photo and Copyright: James Hamblen
Site of the author
Used with permission of the author
Galerie de photos:



 ...
...  Accès à la galerie complète (42 images au total)
Accès à la galerie complète (42 images au total)
Vous avez une photo de ce minéral que vous voudriez voir figurer dans la galerie? Contactez-nous!
Phosphorescence (au sens commun du terme) observable à l'oeil nu:
Type d'UV |
Couleur |
Intensité |
Fréquence d'observation |
|---|---|---|---|
UV longs (365nm): | Aucune perceptible par l'oeil | UV courts (254 nm): | Aucune perceptible par l'oeil |
Activateur(s) et spectre(s):
Activateur(s): TiO6, Cr3+, Fe3+, Ti3+, Ti4+, Cu+, Mn4+, Ti - O,
Pics dans le spectre (nm):
TiO6 : broad band at 419nm (due to the transition of 3T1 → 1A1 in the TiO6 octahedra)
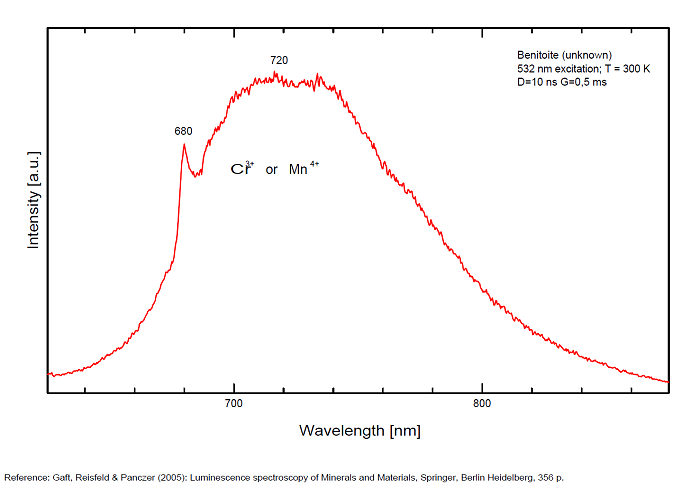
Ti3+ : broad band around 650-660nm with half-width of +/-135nm
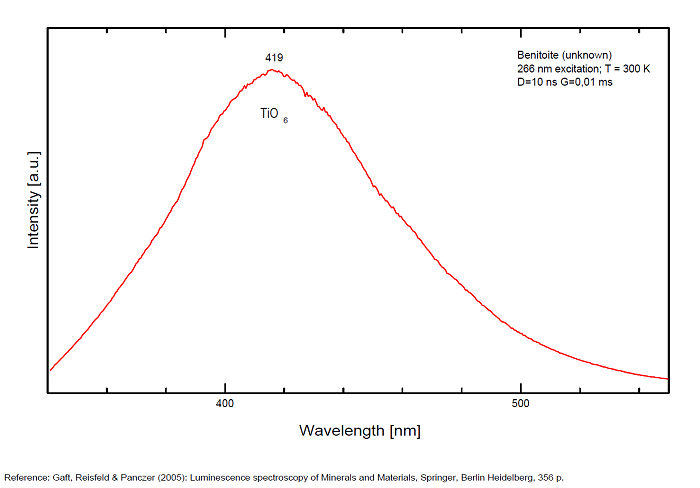
Cr3+ : peak at 680nm
Mn4+ : large band at 720nm
Cu+? replacing Ba2+ and Ti3+? (exc 337nm:(Gorobets)): 720nm
450nm (exc 254nm)
Spectrum: Michael Gaft, Petah Tikva, Israel. Plot: Institute of Mineralogy, University of Vienna, Austria, with permission of the authors.
Galerie de spectres:

 ...
...  Accès à la galerie complète (3 images au total)
Accès à la galerie complète (3 images au total)
Commentaires sur les spectres et les activateurs (*):
Robbins cites Fe as activator for the red color and Ti for the blue; recent spectrographic studies give new data: Decay time shortening and thermal quenching are connected with nonradiative decay within the TiO6 luminescence center, while energy migration does not take place at least up to room temperature.
There are three broadbands:
- one around 419 nm (blue band) is connected with intrinsic luminescence centers, namely isolated TiO6 octahedra,
- one at 650 nm (Yellow band) for Ti3+ ,
- one at 720 (half-width 125nm) for Mn4+
and a narrow line at 680 for Cr3+.(Gaft)
Manganese participation is supported by chemical analysis of benitoite (0.03 to 0.11%).
The metastable level 3T1u is the emitting level at low temperatures with a long decay time of 1.1 ms. At higher temperatures an energy level with higher radiation probability must be involved in the emission process, and this level is situated at 0.06 eV higher than the lowest level. These two levels may be connected with 3T1u level splitting or with closely spaced 3T1u and 3T2u levels.
(Source: M. Gaft, L. Nagli, G. Waychunas and D. Weiss, The nature of blue luminescence from natural benitoite, Physics and Chemistry of Minerals, vol31, 2004)
The red emission of benitoite consists of two individual bands and one line and suggest that the activators of luminescence in benitoite system are Ti3+ and a d3 element, namely Cr3+ or Mn4+.
(Source: M. Gaft, L. Nagli, G. Waychunas and G. Panczer, The nature of red luminescence of natural benitoite, Mineralogy and Petrology, Vol85, 2005)
(*)Les commentaires sur les spectres sont uniquement rédigés en Anglais
Meilleure(s) localité(s) pour la fluorescence (*):
- Benitoite Gem Mine, San Benito County, California, USA;
- Big Creek and Rush Creek junction, Fresno County, California, USA;
- Esquire #1 Claim, Rush Creek, Fresno County, California, USA;
- Victor Mine, San Benito County, California, USA;
- Kinzandani, Hashidate, Ohmi, Itoigawa City, Niigata Prefecture, Chubu Region, Honshu Island, Japan;
(*)Les données ne sont pas exhaustives et sont limitées à quelques localités remarquables pour la fluorescence
Référence bibliographique pour la luminescence:
- The Henkel Glossary of Fluorescent Minerals, Dr. Gerhard Henkel, Published by the FMS, 1989 ,
- Fluorescence: Gems and Minerals Under Ultraviolet Light, Manuel Robbins, 1994, Geoscience Press, ISBN 0-945005-13-X ,
- The World of Fluorescent Minerals, Stuart Schneider, Schiffer Publishing, 2006, ISBN 0-7643-2544-2 ,
- Luminescence Spectroscopy of Minerals and Materials, M. Gaft, R. Reisfeld, G. Panczer, Springer Editor, ISBN: 10 3-540-21918-8 ,
- Luminescent Spectra of Minerals, Boris S. Gorobets and Alexandre A. Rogojine, Moscow, 2002 ,
Référence pour la luminescence sur internet:
- The nature of blue luminescence from natural benitoite, M. Gaft, L. Nagli, G. Waychunas and D. Weiss, Physics and Chemistry of Minerals, vol31, 2004
- The nature of red luminescence of natural benitoite, M. Gaft, L. Nagli, G. Waychunas and G. Panczer, Mineralogy and Petrology, Vol85, 2005
- Benitoite Gem mine (Mindat.org)
Référence minéralogique sur internet:
 http://www.mindat.org/show.php?name=Benitoite
http://www.mindat.org/show.php?name=Benitoite
 http://webmineral.com/data/Benitoite.shtml
http://webmineral.com/data/Benitoite.shtml
Recherche sur Internet:
 Recherche d'images sur 'Google Image'
Recherche d'images sur 'Google Image'
 Recherche de documents en français sur Google
Recherche de documents en français sur Google
 Recherche de documents en toutes
langues sur Google
Recherche de documents en toutes
langues sur Google
Une requête ne donnant pas de résultat signifie uniquement qu'aucune référence de ce type n'existe dans la base mais en aucun cas qu'elle n'existe pas dans l'absolu. Si vous considérez avoir trouvé une erreur ou une omission, merci de nous le signaler via la page contact en n'oubliant pas de citer la source de l'information.