Base de données des minéraux luminescents
SODALITE
Formule chimique: Na8Al6Si6O24Cl2
Famille: Silicates
Statut: IMA-GP
Système cristallin : Cubique
Minéral de vitrine: OUI
Noms associés (variétés luminescentes, noms discrédités, synonymes etc.): hackmanite, yooperlite,
Luminescence:
Couleurs UV longs (365nm): |
Orange , Blanc jaunâtre (crème) , Jaune-orange , Rouge , Rouge violacé , Rose violacé , Rose , | ||
Intensité OL:Très forte | Fréquence OL:Souvent | ||
Couleurs UV courts (254nm): |
Blanc jaunâtre (crème) , Orange , Rouge violacé , Rose violacé , Rose saumon (rose orange) , Vert , | ||
Intensité OC:Moyenne | |||
Photo en lumière du jour
Photo Ondes longues (365nm)
Photo Ondes Courtes (254nm)
Galerie de photos:

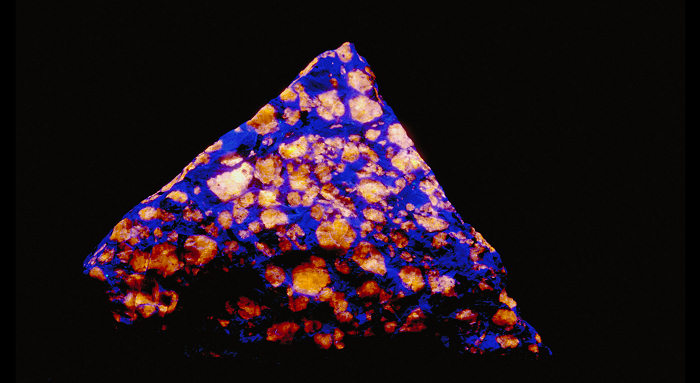
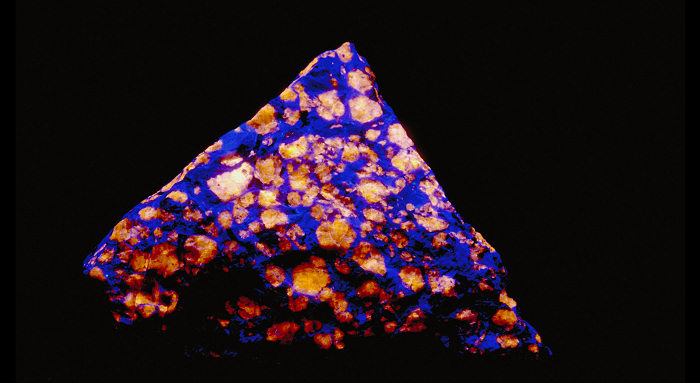
 ...
...  Accès à la galerie complète (21 images au total)
Accès à la galerie complète (21 images au total)
Vous avez une photo de ce minéral que vous voudriez voir figurer dans la galerie? Contactez-nous!
Phosphorescence (au sens commun du terme) observable à l'oeil nu:
Type d'UV |
Couleur |
Intensité |
Fréquence d'observation |
|---|---|---|---|
UV longs (365nm): | Blanc bleuté | Forte | Souvent | UV courts (254 nm): | Blanc bleuté | Très forte | Souvent |
Ténébrescence: OUI

Sodalite, left before and right after exposition to SW (tenebrescence);
Ilimaussaq Complex, Greenland;
Courtesy MinerShop.com
Thermoluminescence: OUI
Commentaires:
HACKMANITE : variété fortement ténébrescente de sodalite. Certaines sodalites du Mont Saint-Hillaire fluorescent en jaune sous les UV ondes courtes et moyennes, plus faiblement en ondes longues. La luminescence met un certain temps avant d arriver à son maximum. Yooperlite: galets de syénite contenant de la sodalite fluorescente trouvée en 2018 par Erik Rintamaki, un marchand de minéraux, sur les plages du lac Supérieur, Michigan, USA (voir Yooperlite).
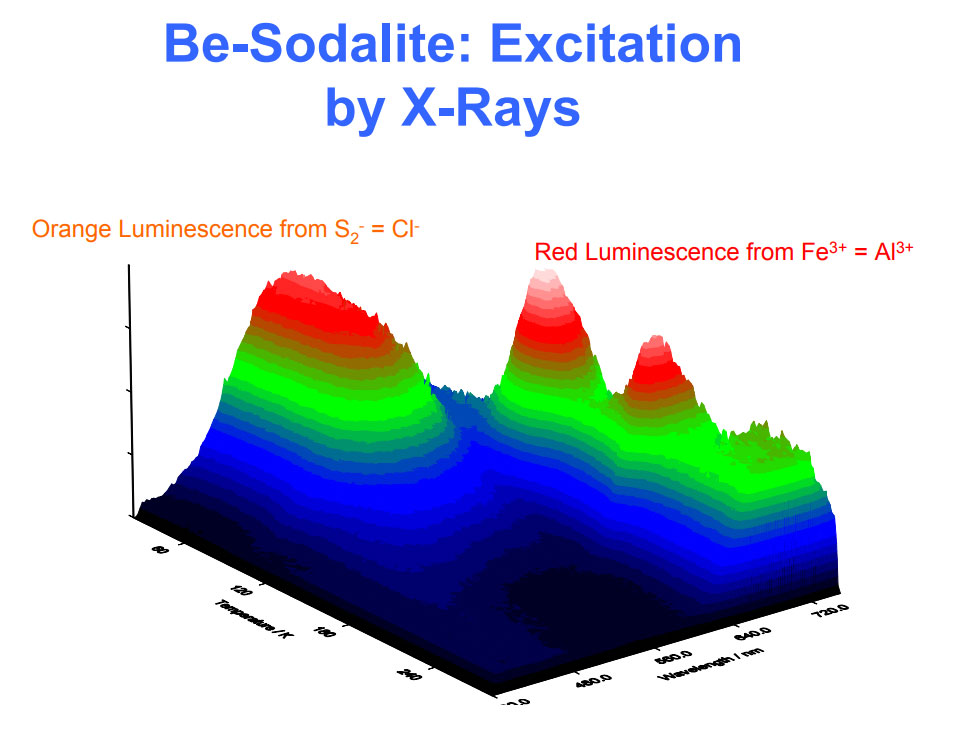
Activateur(s) et spectre(s):
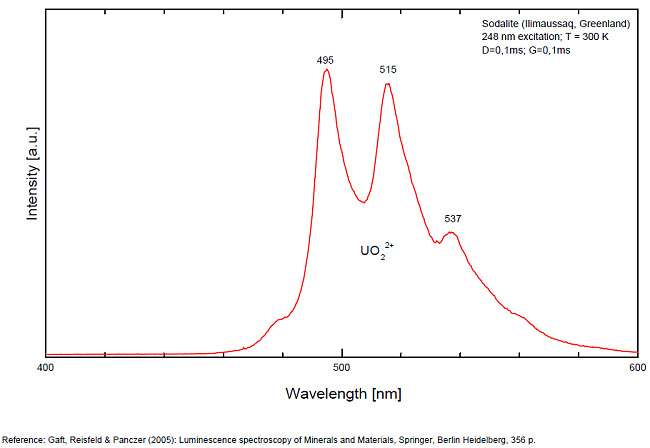
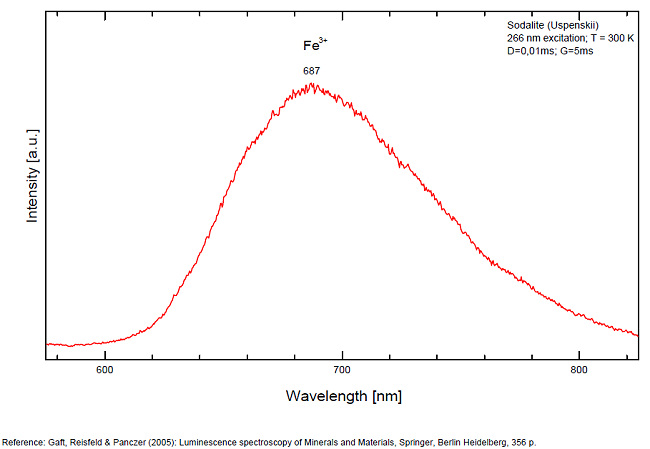
Activateur(s): S2-, (UO2)2+ (ion Uranyle) en impureté, Fe3+, Mn2+ ,
Pics dans le spectre (nm):
S2- : 587, 608, 628, 653, 677, 707, 732nm Fe3+? repl. Al3+ : 687-720nm (UO2)2+ : 495, 515, 537nm Mn2+ repl. Na+ : 650nm
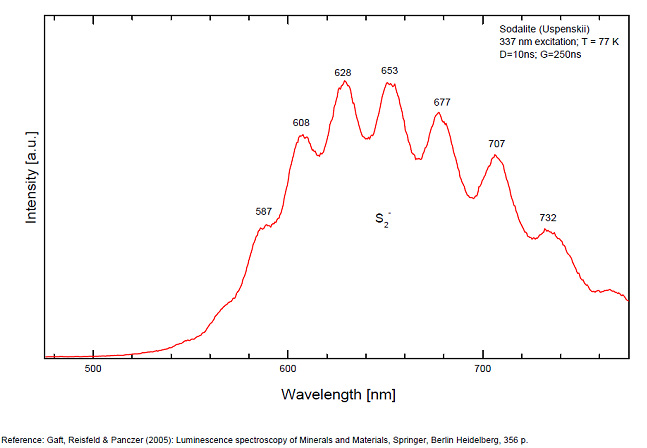
Spectrum: Michael Gaft, Petah Tikva, Israel. Plot: Institute of Mineralogy, University of Vienna, Austria, with permission of the authors.
Galerie de spectres:

 ...
...  Accès à la galerie complète (3 images au total)
Accès à la galerie complète (3 images au total)
Commentaires sur les spectres et les activateurs (*):
Some time Green Luminescence due to uranium impurities; Cathodoluminescence: intensive greenish-blue or yellow-green or orange. The research history of the yellow-orange luminescence of sodalite was well presented by Sidike et al (2007). It may be summarized that published data on the yellow-orange luminescence spectra of sodalite was ascribed to S2- which was confirmed by the study of synthetic analogs activated by sulfur. Nevertheless, Sidike et al. (2007) noted that the peak wavelengths in the yellow orange band reported by many investigators for sodalite and ascribed to S2-do not agree. It was never reported by single author on several types of S2- luminescence spectra from the same deposit, which may be explained by different chemical composition and impurities, but different spectra were ascribed to the S2- luminescence center in sodalite samples from the same deposit. The values for luminescence maxima were reported to range from 630 nm to 708 nm. A similar situation takes place in scapolite, where the peak wavelengths of S2- emission bands reported by different authors do not agree (Sidike et al. 2008). It was proposed that the main reason for these discrepancies is probably the unsuitable correction of the measured spectra. Nevertheless, it is difficult to assume that such strong discrepancies may be explained only by the absence of proper calibration. A possible explanation for the differences between published spectra may be the presence of several different S2- centers, or the presence of the luminescence centers with vibrational structure of other types. (Gaft) The crystal structure of sodalite is an ordered framework of linked AlO4 and SiO4 tetrahedra in which Si and Al alternate on the tetrahedral sites. The overall linkage of (Al,Si)O4 tetrahedra results in cubo-octahedral cavities, which contain a centrally placed anion coordinated tetrahedrally to four cations. The flexibility of the sodalite structure allows a wide range of cations and anions with potential luminescence ability to be substituted into it. Thus, many impurities with potential luminescence ability besides S2- may be present in the sodalite structure, such as Fe3+ in Al and Si positions, Mn2+ in Na or Be positions, Pb2+ or Tl+ in the Na position and so on. (Gaft) By Laser-induced time-resolved technique Gaft was able to detect two Fe3+ or Cr3+ (broad band at 687 - 700 & 725 nm), possibly Pb2+ (broad band at 440nm), Eu2+ (band at 405nm), Ce3+ ( band at 340nm) and S2- (broad band at 600 - 620 - 653 - 655nm with weak vibrational structure) emission centers (Gaft) The photoluminescence and excitation spectra of sodalites from Greenland, Canada and Xinjiang (China) are observed at 300 and 10 K in detail. The features of the emission and excitation spectra of the orange-yellow fluorescence of these sodalites are independent of the locality. The emission spectra at 300 and 10 K consist of a broad band with a series of peaks and a maximum peak at 648 and 645.9 nm, respectively. The excitation spectra obtained by monitoring the orange-yellow fluorescence at 300 and 10 K consist of a main band with a peak at 392 nm. The luminescence efficiency of the heat-treated sodalite from Xinjiang is about seven times as high as that of untreated natural sodalite. The emission spectrum of the S2 − center in sodalite at 10 K consists of a band with a clearly resolved structure with a series of maxima spaced about 560 cm−1 (20–25 nm) apart. Each narrow band at 10 K shows a fine structure consisting of a small peak due to the stretching vibration of the isotopic species of 32S34S−, a main peak due to that of the isotopic species of 32S2 − and five peaks due to phonon sidebands of the main peak. (see Aierken Sidike, Alifu Sawuti, Xiang-Ming Wang, Heng-Jiang Zhu, S. Kobayashi, I. Kusachi, N. Yamashita, Fine structure in photoluminescence spectrum of S2− center in sodalite, Physics and Chemistry of Minerals, September 2007, Volume 34, Issue 7, pp 477–484 ) The emission and excitation spectra of yellow luminescence due to S2− in scapolites (from Canada and from an unknown locality) were observed at 300, 80 and 10 K. Emission and excitation bands at 10 K showed vibronic structures with a series of maxima spaced 15–30 and 5–9 nm, respectively. The relative efficiency of yellow luminescence from scapolite #2 was increased up to 117 times by heat treatment at 1,000°C for 2 h in air. The enhancement of yellow luminescence by heat treatment was ascribed to the alteration of SO32− and SO4 2− to S2 − in scapolite. (see Aierken Sidike, I. Kusachi, S. Kobayashi, K. Atobe, N. Yamashita, Photoluminescence spectra of S2− center in natural and heat-treated scapolites, Physics and Chemistry of Minerals, April 2008, Volume 35, Issue 3, pp 137–145 )
(*)Les commentaires sur les spectres sont uniquement rédigés en Anglais
Meilleure(s) localité(s) pour la fluorescence (*):
- Brevik, Langesundfjord, Porsgrunn, Telemark, Larvik Plutonic Complex, Norway ;
- Bancroft, Ontario, Canada ;
- Khibiny Massif, Kola Peninsula, Murmanskaja Oblast, Northern Region, Russia ;
- Ilimaussaq complex, Kvanefjeld, Narsaq, Kitaa (West Greenland) Province, Greenland ;
- Kangerluarsuk Fjord (Kangerdluarssuq Fjord), Ilímaussaq complex, Narsaq, Kujalleq, Greenland ;
- Tunulliarfik Fjord, Ilímaussaq complex, Narsaq, Kujalleq, Greenland, Denmark;
- Kiran, Kokcha (Koksha) Valley, Badakhshan Province, Afghanistan ;
- Sar-e-Sang, Kokcha (Koksha) Valley, Badakhshan Province, Afghanistan ;
- Mont Saint-Hilaire, La Vallée-du-Richelieu RCM, Montérégie, Québec, Canada (bright orange under LW & dull orange SW);
- Symmes Pond Syenite, Straw Hill, Newfield, York Co., Maine, USA (yellow SW, orange-yellow LW);
- Horne Quarry, Red Hill, Moultonborough, Carroll Co., New Hampshire, USA (strong orange LW);
- AkeSu Area, Xinjiang Autonomous Region, China (Blue sodalite fluo LW red-orange + SW green);
- Beemerville main nepheline-syenite mass, Libertyville, Wantage Township, Sussex County, New Jersey, USA;
- Salem, Essex Co., Massachusetts, USA;
- Horne Quarry, Red Hill, Moultonborough, Carroll Co., New Hampshire, USA;
- Symmes Pond Syenite, Straw Hill, Newfield, York Co., Maine, USA (bright yellow SW & more orange yellow LW);
- Ice River Alkaline Complex, Golden Mining Division, British Columbia, Canada (The grey sodalite fluoresces brilliant orange, but not the blue);
- Cancrinite Hill, Dungannon Township, Hastings County, Ontario, Canada (associated with Cancrinite, SW dark red fluorescence);
- Lake Superior beaches, Chippewa Co., Michigan, USA (Yooperlite);
(*)Les données ne sont pas exhaustives et sont limitées à quelques localités remarquables pour la fluorescence
Référence bibliographique pour la luminescence:
- The Henkel Glossary of Fluorescent Minerals, Dr. Gerhard Henkel, Published by the FMS, 1989 ,
- Fluorescence: Gems and Minerals Under Ultraviolet Light, Manuel Robbins, 1994, Geoscience Press, ISBN 0-945005-13-X ,
- The World of Fluorescent Minerals, Stuart Schneider, Schiffer Publishing, 2006, ISBN 0-7643-2544-2 ,
- Luminescence Spectroscopy of Minerals and Materials, M. Gaft, R. Reisfeld, G. Panczer, Springer Editor, ISBN: 10 3-540-21918-8 ,
- Luminescent Spectra of Minerals, Boris S. Gorobets and Alexandre A. Rogojine, Moscow, 2002 ,
- Mt St Hilaire Website: http://www.saint-hilaire.ca ,
- Minershop.com ,
- Luminescenza nel regno minerale, Guido Mazzoleni, fotografia Roberto Appiani, Libri Sandit, 2010, ISBN 978-88-95990-63-7 ,
Référence pour la luminescence sur internet:
- Article on hackmanite in Gems and gemmology, Spring 2009
- Fluorescent minerals of Greenland from The Minershop
- www.minershop.com/FLmingreen.pdf (Pdf,1,54 Mb)
- www.minershop.com/history.pdf (Pdf, 22Mb)
- http://www.minsocam.org/ammin/AM40/AM40_22.pdf
- The Langesundsfjord: history, geology, pegmatites, minerals, Alf Olav Larsen, Bode Verlag Gmbh, 2010 ISBN 978-3-925094-97-2
- A. Hassi, O.Beckman Annersten, Photochromic properties of natural sodalite , J.Phys.D.App.Phys.10, 771 (1977)
- Article sur la thermoluminescence de la sodalite: http://iopscience.iop.org/1742-6596/249/1/012022/pdf/1742-6596_249_1_012022.pdf
- MONT-SAINT-HILAIRE, History, Geology, Mineralogy, Laszlo HORVATH, The Canadian Mineralogist, Special Publication 14, 2019
Images:
- Ténébrecence Greenland: http://www.mindat.org/photo-128309.html
- Tunulliarfik Fjord, Ilímaussaq complex, Greenland LW : https://www.mindat.org/photo-857126.html
- Tunulliarfik Fjord, Ilímaussaq complex, Greenland SW : https://www.mindat.org/photo-857127.html
- Tunulliarfik Fjord, Ilímaussaq complex, Greenland Daylight, LW, SW: https://www.mindat.org/photo-857129.html
- Beemerville, New Jersey, USA : https://www.mindat.org/photo-33878.html
- Salem, Essex Co., Massachusetts, USA : https://www.mindat.org/photo-135587.html
- Horne Quarry, New Hampshire, USA : https://www.mindat.org/photo-177047.html
- Mont Saint-Hilaire, Québec, Canada : https://www.mindat.org/photo-367544.html
- Sar-e-Sang, Afghanistan : https://www.mindat.org/photo-431664.html
- Yooperlite, Lake Superior beaches, Michigan, USA: https://www.mindat.org/photo-909354.html
Videos:
- sodalite: https://www.instagram.com/p/B1OtkBknNI6
- sodalite tenebrescence: https://www.youtube.com/watch?v=TuHoXjr4g38
- The story of yooperlite: https://www.youtube.com/watch?v=KxOIyheHXMo
- Ténébrescence hackmanite & tugtupite : https://www.youtube.com/watch?v=stDh8GDkEYc
- Ténébrescence Hackmanite Mogok : https://youtu.be/k9B7Omm0nmk
- Ténébrescence hackmanite : https://www.instagram.com/p/BMAb0UWD0nn
Référence minéralogique sur internet:
 http://www.mindat.org/show.php?name=Sodalite
http://www.mindat.org/show.php?name=Sodalite
 http://webmineral.com/data/Sodalite.shtml
http://webmineral.com/data/Sodalite.shtml
Recherche sur Internet:
 Recherche d'images sur 'Google Image'
Recherche d'images sur 'Google Image'
 Recherche de documents en français sur Google
Recherche de documents en français sur Google
 Recherche de documents en toutes
langues sur Google
Recherche de documents en toutes
langues sur Google
Une requête ne donnant pas de résultat signifie uniquement qu'aucune référence de ce type n'existe dans la base mais en aucun cas qu'elle n'existe pas dans l'absolu. Si vous considérez avoir trouvé une erreur ou une omission, merci de nous le signaler via la page contact en n'oubliant pas de citer la source de l'information.