Database https://www.fluomin.org
Atlas of luminescent minerals spectral data
Gérard Barmarin
List of spectra and activator data for listed luminescent minerals
It is specially designed to print out correctly;
There are about 320 sheets in all!
There are 174 spectra of luminescent minerals in the database.
A request providing no result means only that no such reference exists in the database, but it does not mean that what you are looking for does not exist, just not to our knowledge. If you think you have found an error or omission, please let us know via the contact page being sure to cite the source of information.
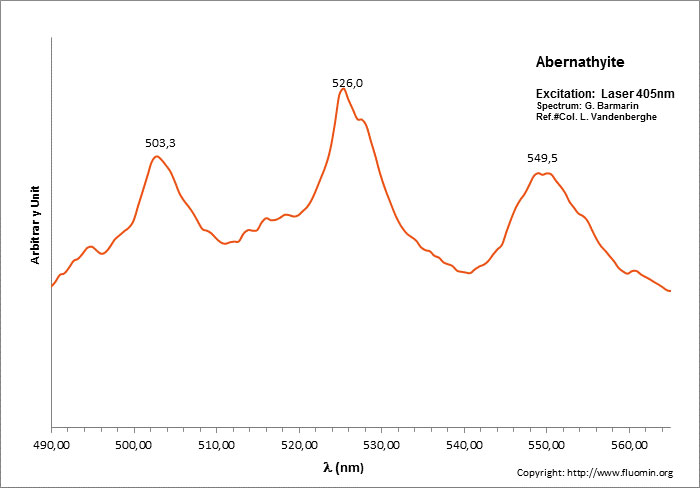
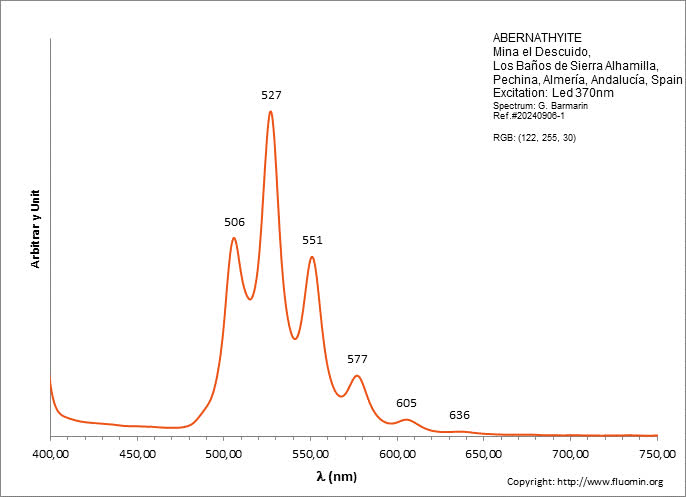
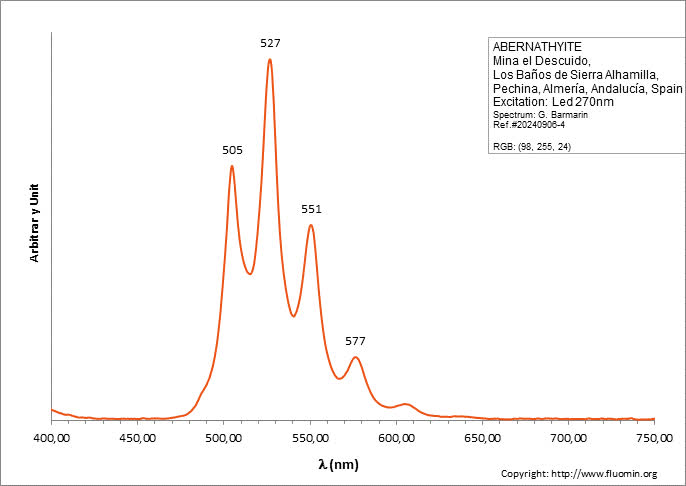
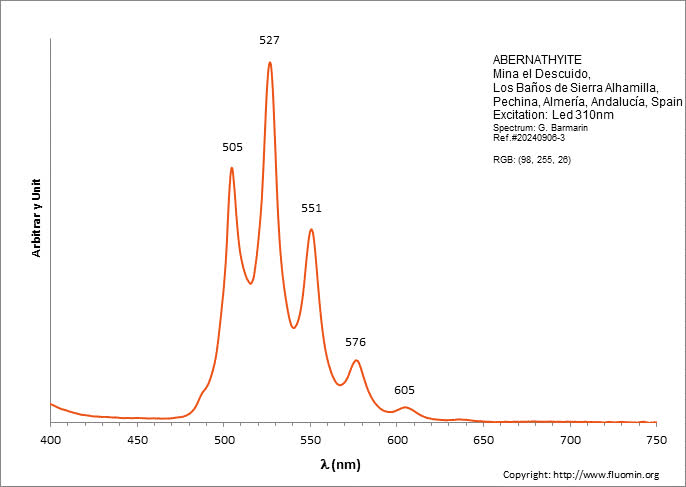
ABERNATHYITEK2(UO2)2(AsO4)2 6H2O
Activator(s):
(UO2)2+ (ion Uranyle) intrinsèque ,
Peaks in the spectrum (nm):
(UO2)2+ : 506, 527, 551, 577, 605, 636nm
Comments on spectra and activators:
0.1 eV (808cm-1) between the peaks. Equivalent RGB color: (106, 255, 26)
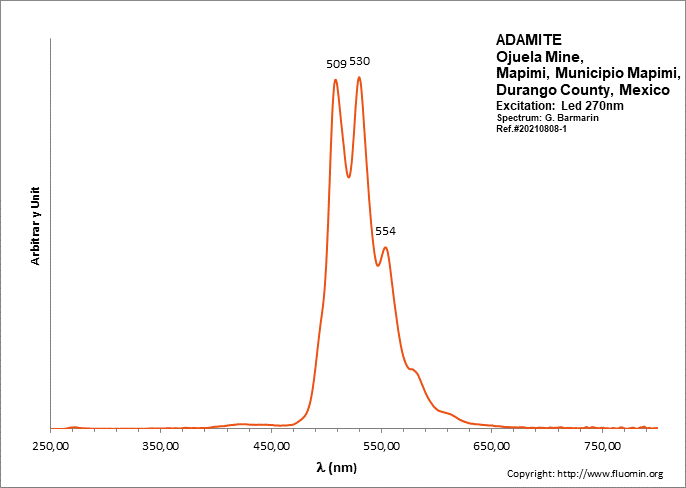
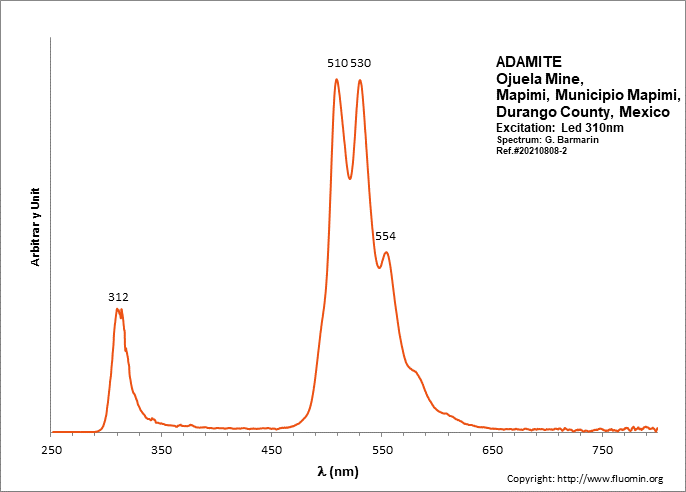
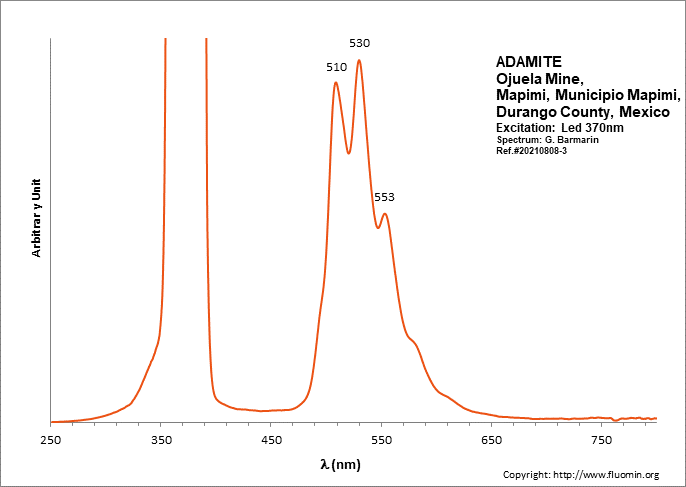
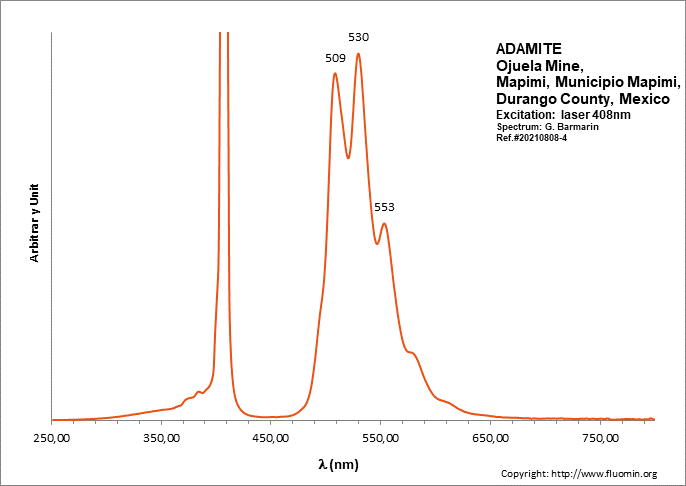
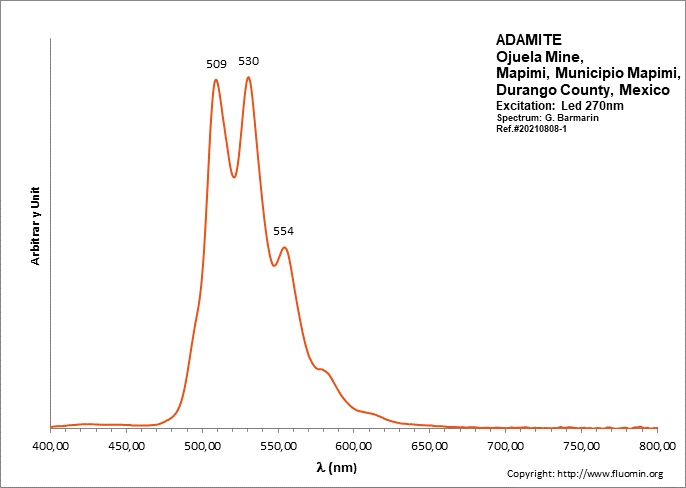
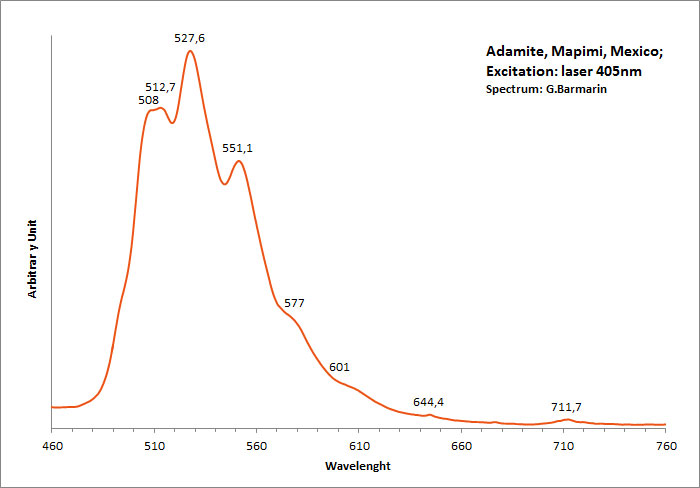
ADAMITEZn2(AsO4)(OH)
Activator(s):
(UO2)2+ (ion Uranyle) en impureté,
Peaks in the spectrum (nm):
UO22+: (496), 508, 513, 529, (539), (550), 553, (560), (578)
Comments on spectra and activators:
Lifetime: 50μs
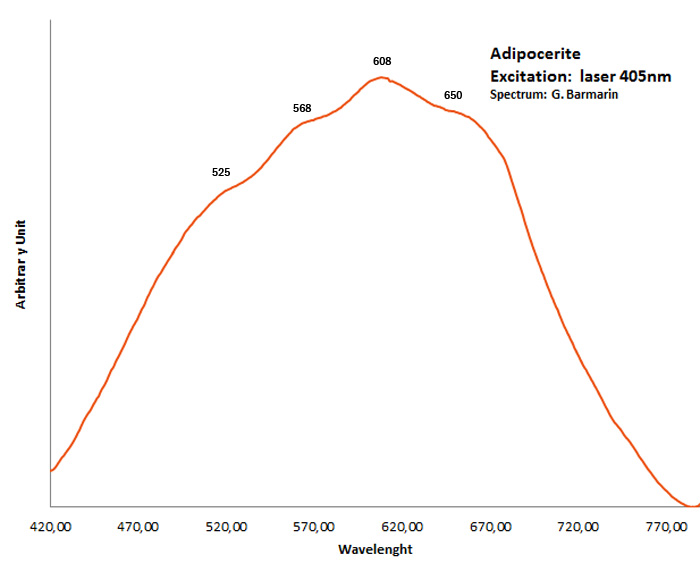
adipocerite
Activator(s):
Matière organique intrinsèque,
Peaks in the spectrum (nm):
very broad band with events at 525, 568, 608, 650nm
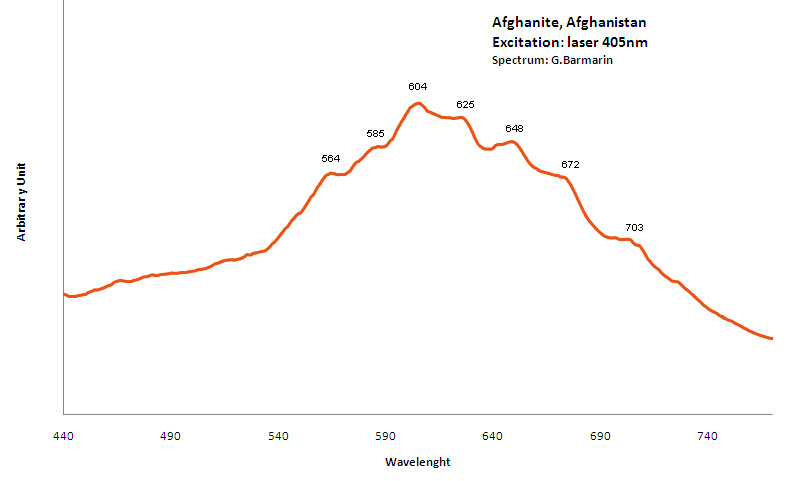
AFGHANITE(Na,Ca,K)8 (Si,Al)12O24 (SO4,Cl,CO3)3 H2O
Activator(s):
S2-,
Peaks in the spectrum (nm):
S2- : 564, 585, 604, 625, 648, 672, 703nm
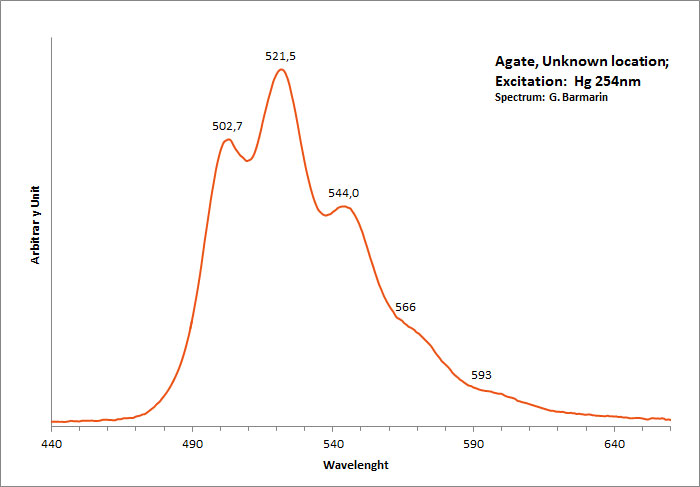
agate
Activator(s):
(UO2)2+ (ion Uranyle) en impureté,
Peaks in the spectrum (nm):
(UO2)2+ : 502,7, 521,5, 544, 566, 593nm
Comments on spectra and activators:
Typical spectrum linked to the presence of uranium ions.
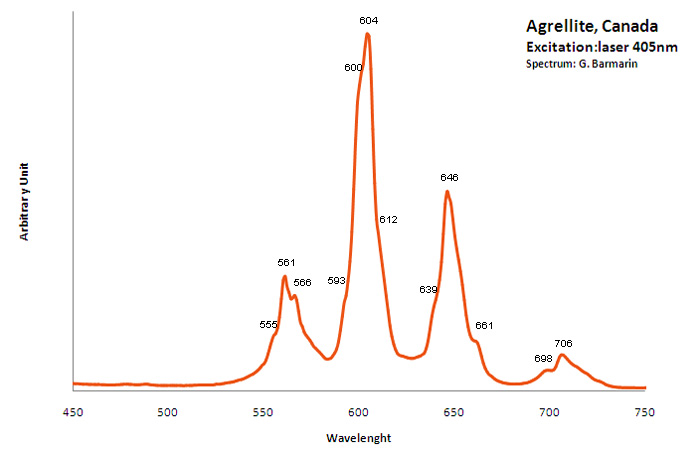
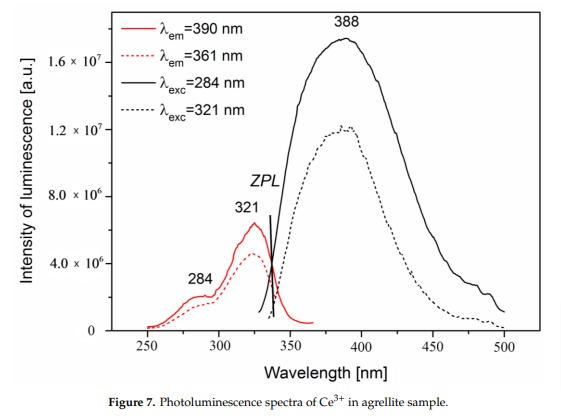
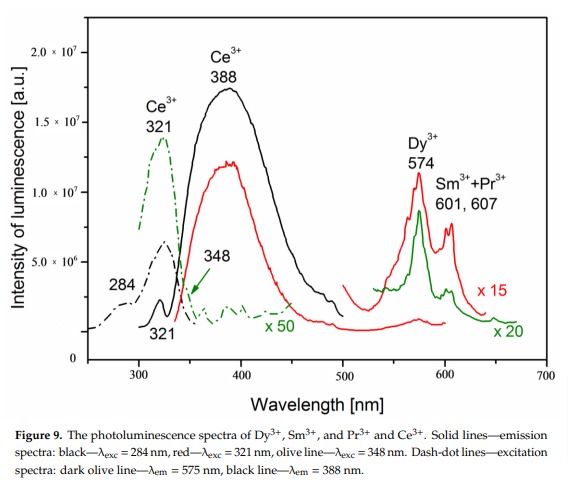
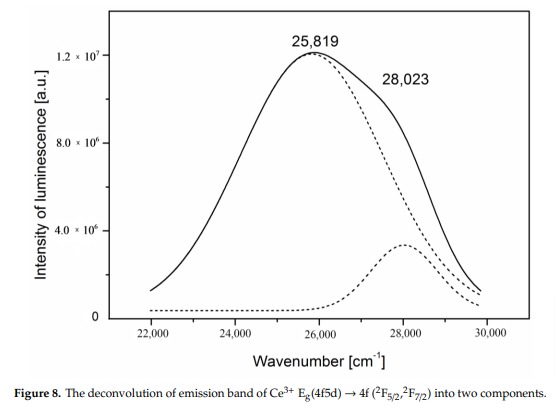
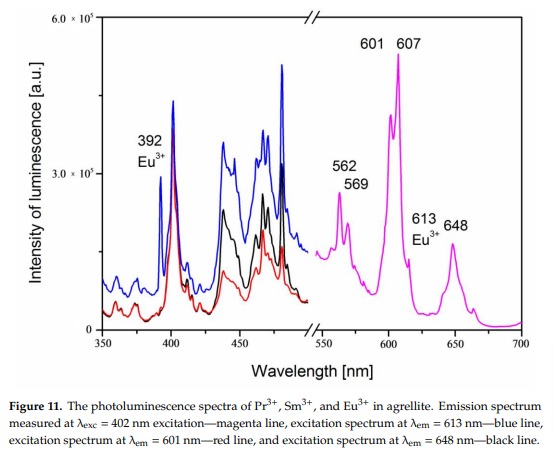
AGRELLITENaCa2Si4O10F
Activator(s):
Mn2+ ,
Other activators:
Fe3+, Eu2+, Ce3+, Sm3+, Dy3+, Nd3+, Gd3+,
Peaks in the spectrum (nm):
Mn2+ replacing Ca2+: Large band peaking at 580nm
Eu2+: 410nm
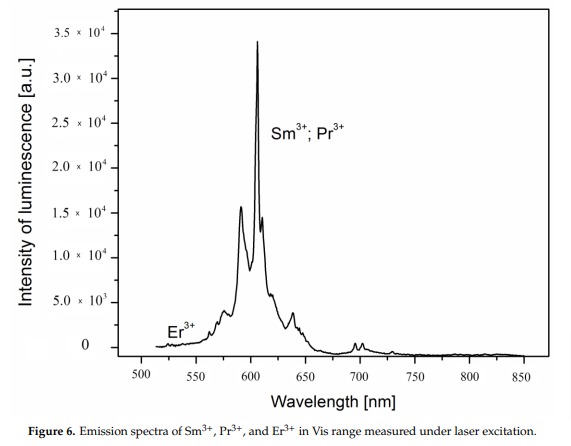
Sm3+: 560, 597, 602, 640nm
Dy3+: 475, 485, 580nm
Fe3+ replacing Si4+: 740nm
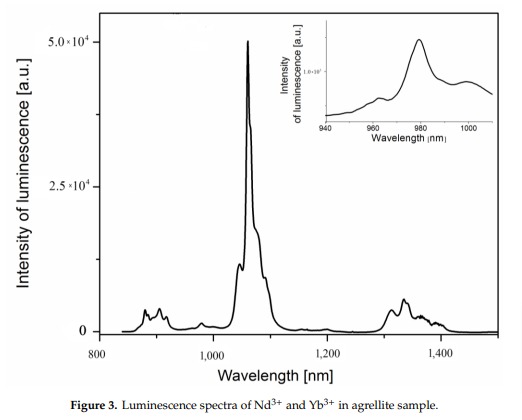
Nd3+(?): 955nm
Ref. sample: (555), (561), 566, (593), (600), 604, (612), (639), 646, 661, 698 and 706 nm
Comments on spectra and activators:
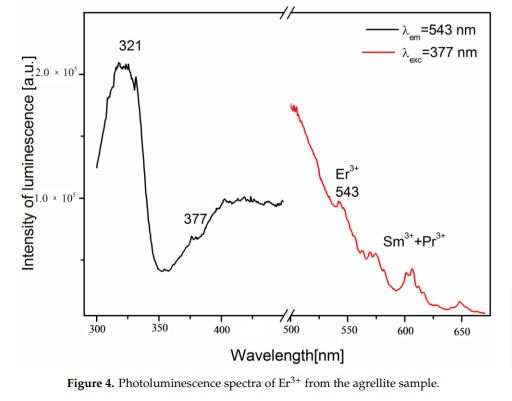
A brief but distinctive orange flash is seen before the magenta luminescence (typical of Mn activator?). There is sometime a green fluorescent mineral accompagning agrellite. It is sometime reported as thorium activated calcite (???) that will glow green sw and mw whitish green lw and will phos under all 3 waves. Sometime thorite coating are invoqued to explain the green fluorescence in specimens from Kipawa. Albite fluorescing red SW is also often present. The emission centers observed in agrellite samples vary depending on their geographical origin. Here is a summary of the findings: Kipawa River Samples: Yakutia Samples: Dara-i-Pioz Samples: Additionally, recent spectroscopic investigations of agrellite samples from Dara-i-Pioz (Tajikistan) and Murun massif (Russia) have shown luminescence from Ce³⁺ and EPR spectra of Mn²⁺. However, it is noted that some expected emissions were not observed in certain samples. For instance, the luminescence of Mn²⁺ and Eu²⁺ was not observed in some specimens
RE elements (RRE2+ and RRE3+) replacing Ca2+ are suspected as activators. Also Mn and Fe.
Fluorescence: pink LW after Robbins and other authors, clearly visible with pointer-laser @405nm.
As with other minerals activated by RRE, the shape of the spectrum is strongly influenced by the resolution of the spectrometer because RRE have a lot of very narrow peaks very close from each other. If the resolution of the spectrometer is not sufficient, the pikes are agglomerated in larger entities.
Minerals 2019, 9, 752; doi:10.3390/min9120752
Minerals 2019, 9, 752; doi:10.3390/min9120752
Minerals 2019, 9, 752; doi:10.3390/min9120752
Minerals 2019, 9, 752; doi:10.3390/min9120752
Minerals 2019, 9, 752; doi:10.3390/min9120752
Minerals 2019, 9, 752; doi:10.3390/min9120752
Minerals 2019, 9, 752; doi:10.3390/min9120752
AGRICOLAITEK4(UO2)(CO3)3
Activator(s):
(UO2)2+ (ion Uranyle) intrinsèque ,
Peaks in the spectrum (nm):
(UO2)2+: (464nm) 484 502 523 545 567nm (594nm)
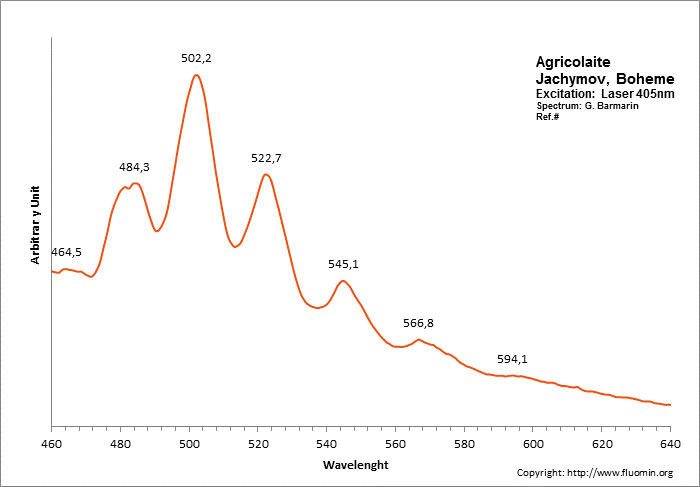
Spectrum: G. Barmarin, Col: Luc Vandenberghe
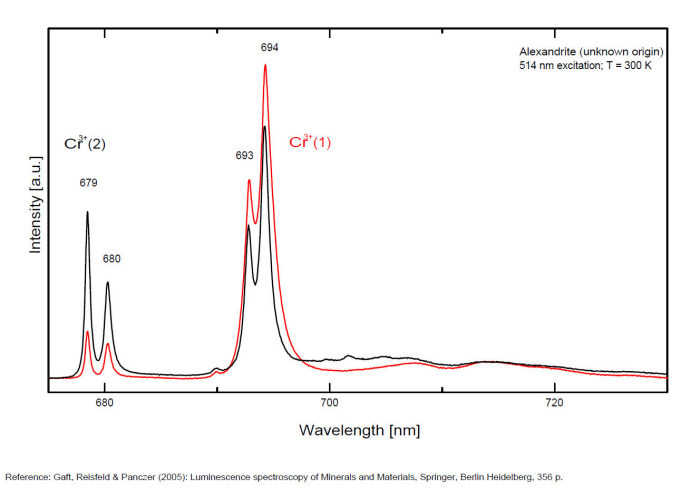
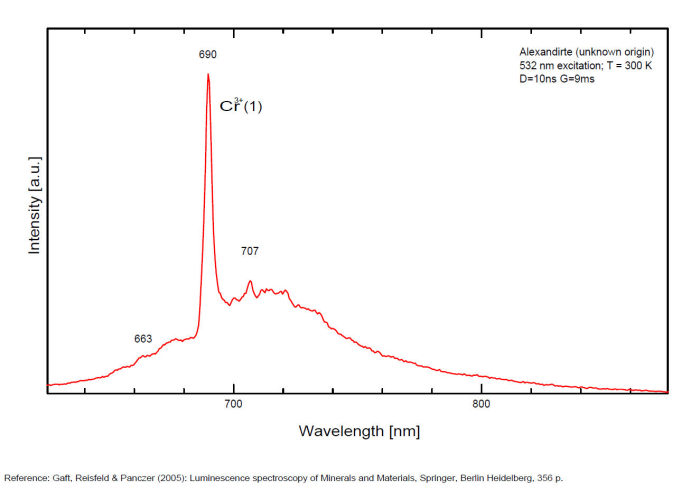
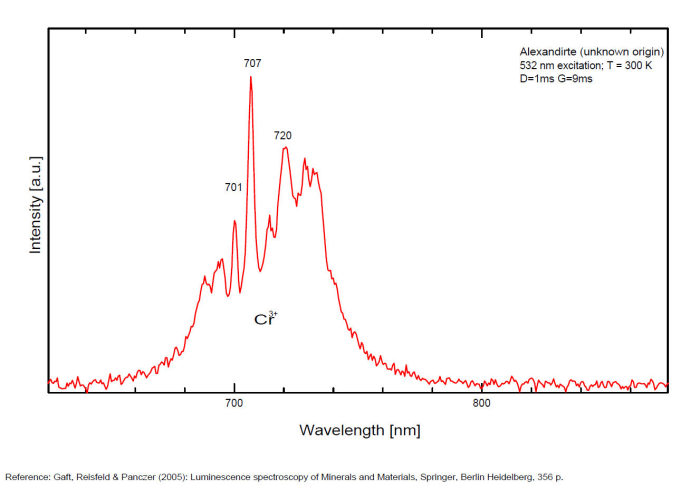
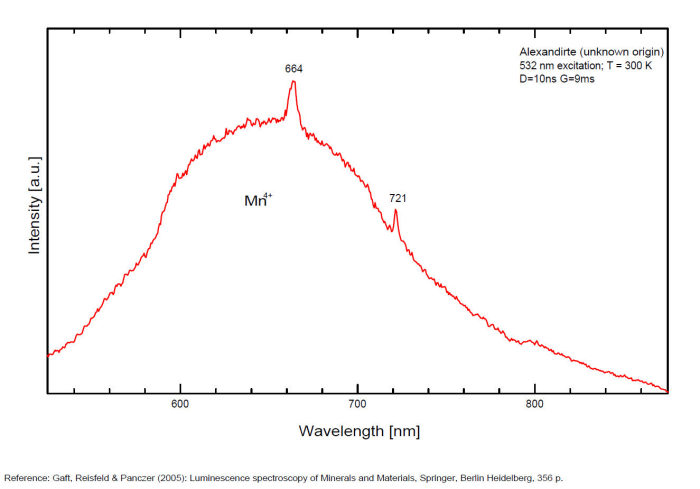
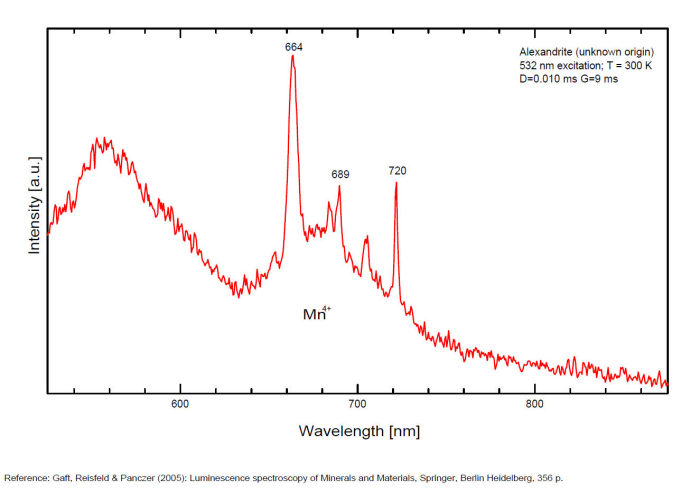
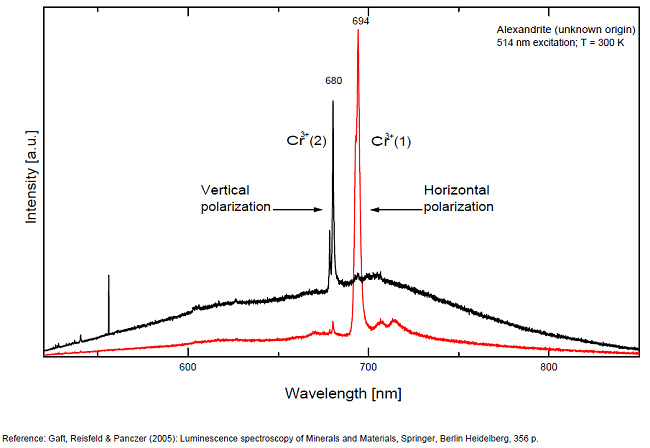
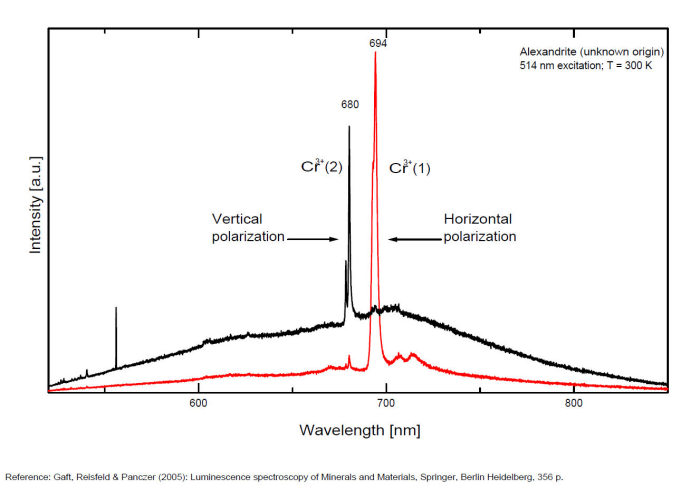
alexandrite
Activator(s):
Cr3+,
Other activators:
Mn4+, V2+,
Peaks in the spectrum (nm):
663, 664, Cr3+ II : 679, 680nm Cr3+ I : 689, 690, 693, 694nm Cr3+ : 701, 707, 720, 721nm Broad band peaking at +/- 600 V2+ (?) : broad band peaking at +/- 717nm Mn4+ : broad bands around 650 - 670nm
Comments on spectra and activators:
Two types of Cr3+ luminescence centers have been found in natural alexandrite, characterized at 300 K by R-lines at approximately 679.0 with 677.3 nm and 694.4 with 691.7 nm, accompanied by very many N-lines of Cr-Cr pairs. Those centers have been identified as connected with substitutions of Al3+ in different structural sites (Tarashchan 1978). (Scalvi et al. 2003; Trindade et al. 2011)
AMBERC12H20O
Activator(s):
Matière organique intrinsèque,
Peaks in the spectrum (nm):
Broad band centered at 500 nm (+/-100nm half-width) / "waves" at 480, 513, 548 and 592nm
The specified directory does not exist.
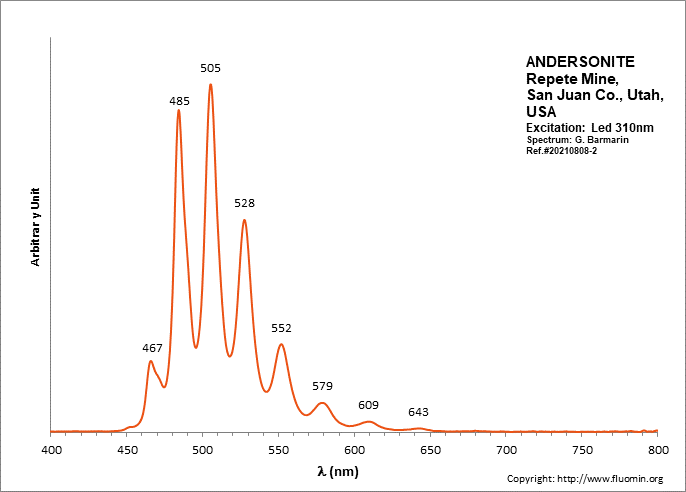
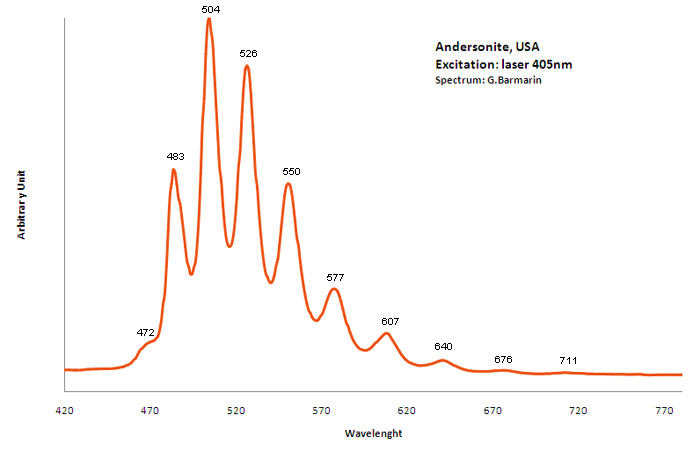
ANDERSONITENa2Ca(UO2)(CO3)3 6H2O
Activator(s):
(UO2)2+ (ion Uranyle) intrinsèque ,
Peaks in the spectrum (nm):
(UO2)2+ : (452),(467), 472, 483,504, 526, 550, 577, 607, 640, (676), (711)
Comments on spectra and activators:
Lifetime: 150μs @500nm;
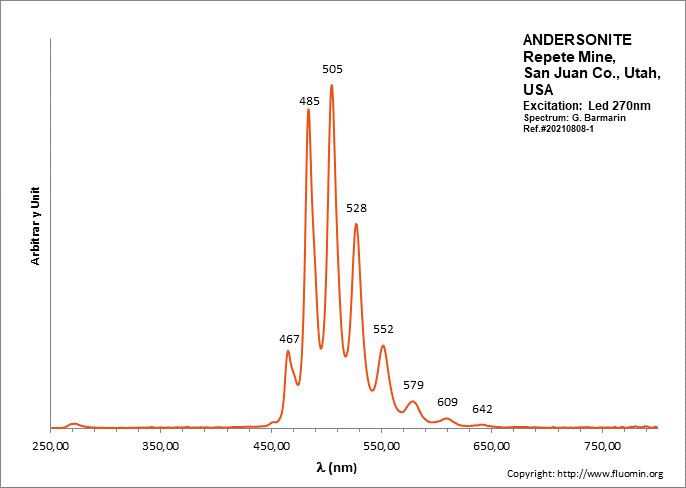
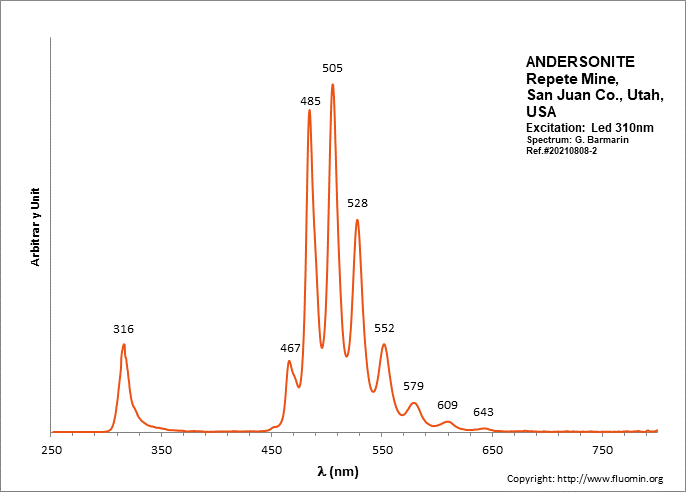
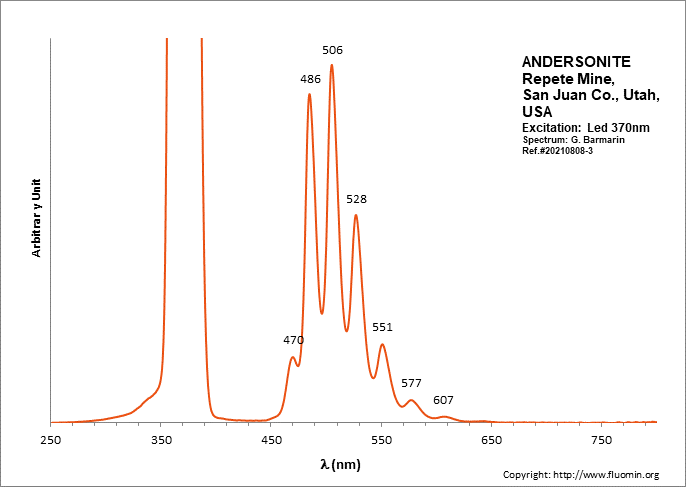
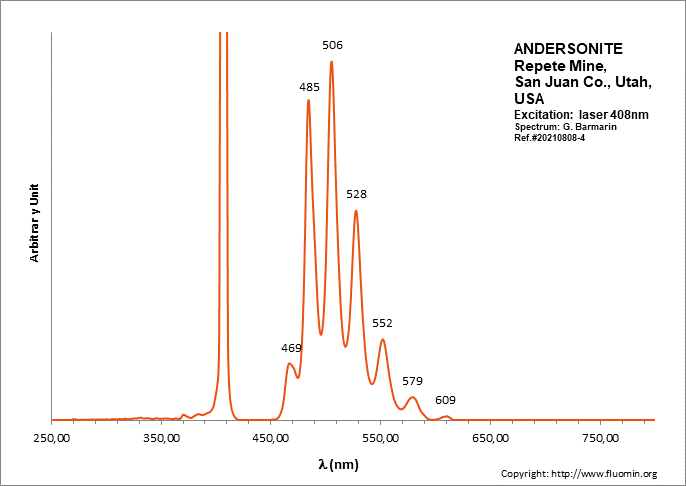
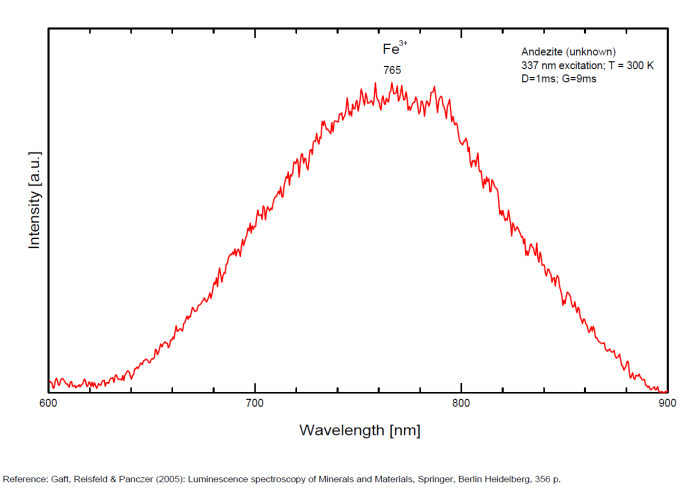
ANDESINE(Na,Ca)(Si,Al)4O8
Activator(s):
Fe3+,
Other activators:
Ce3+, Eu3+,
Peaks in the spectrum (nm):
Ce3+: broad band peaking at 325 nm
Eu3+: broad band peaking at 407nm
Fe3+: broad band at 765nm (130nm half-width)
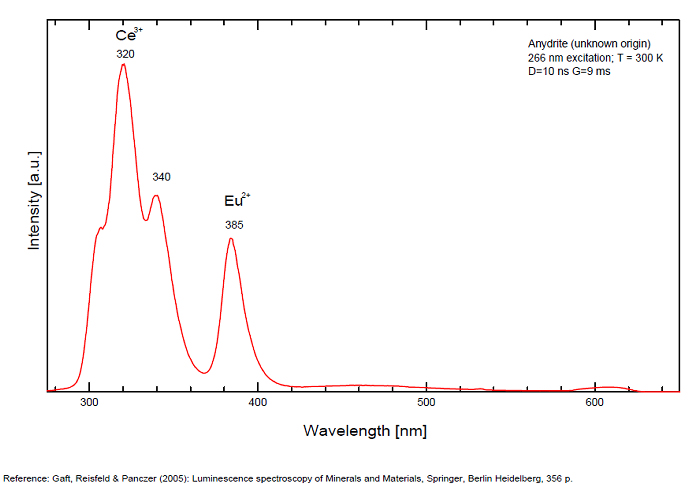
ANHYDRITECaSO4
Activator(s):
Sm2+,
Other activators:
S2-, Eu2+, Ce3+, Eu3+, Dy3+, Tb3+, Mn2+ , O*, Nd3+, Yb2+,
Peaks in the spectrum (nm):
Mn2+II, NOT repl. Ca2+: 600-630nm
O2- repl. [SO4]2-: 400-500nm
S2-: 500-600nm (in N-liquid)
Ce3+: 320, 340nm
Eu2+: 385nm
Tb3+: 416, 438nm
Dy3+: 475, 478, 480, 546, 574, 577, 582nm
Sm2+: 630, 632, 633, 637, 640, 680, 687, 689, 690, 693, 696, 699, 700, 703, 704, 706, 706, 708, 727, 732, 735, 740, 744, 745, 748, 765, 766, 767, 769, 777, 782, 818, 820, 821, 832nm
Nd3+: 838, 839, 892, 899nm
Eu3+: 593, 618, 691, 702nm
Yb2+: 525nm
Comments on spectra and activators:
Activator: Mn2+ or REE substituted to Ca (Gaft); O-2 (Gorobets);
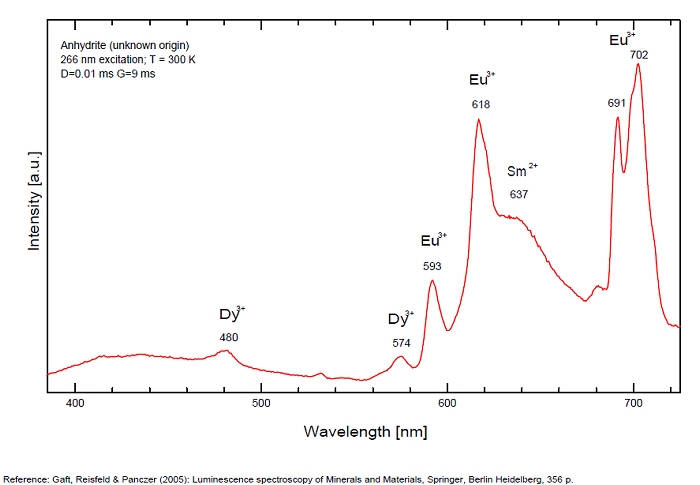
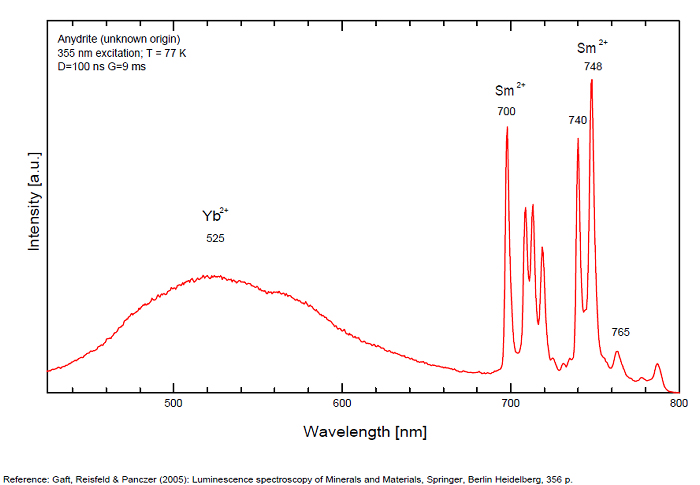
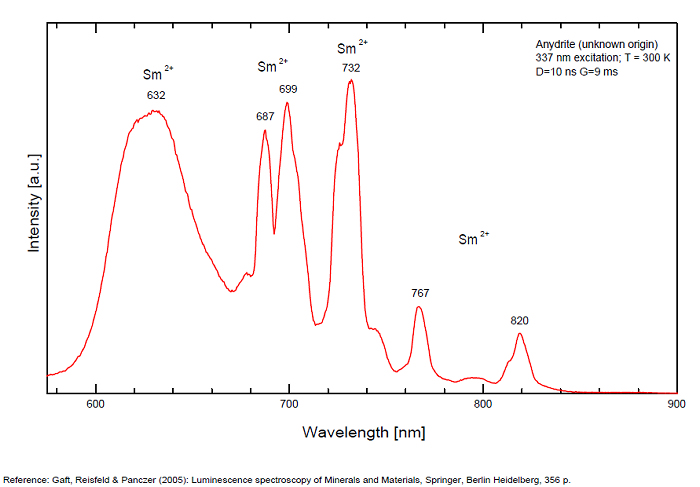
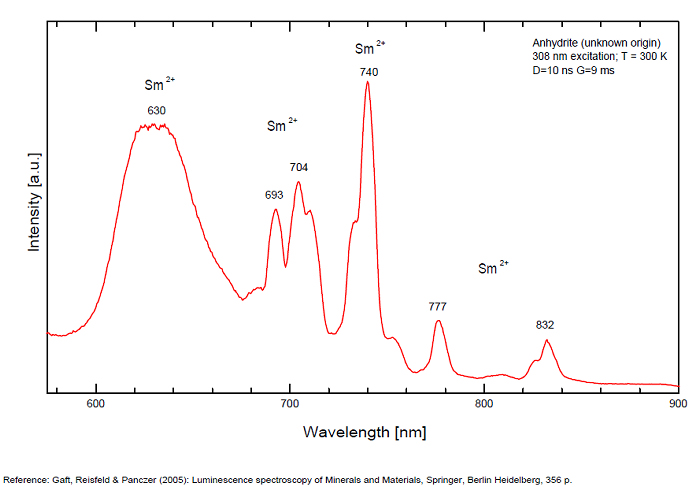
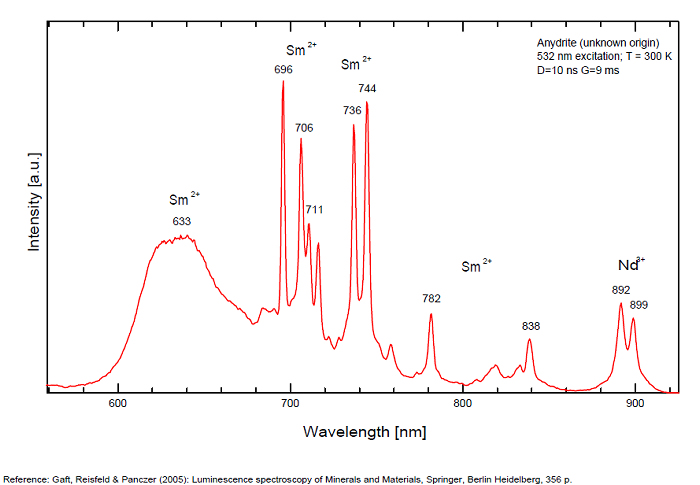
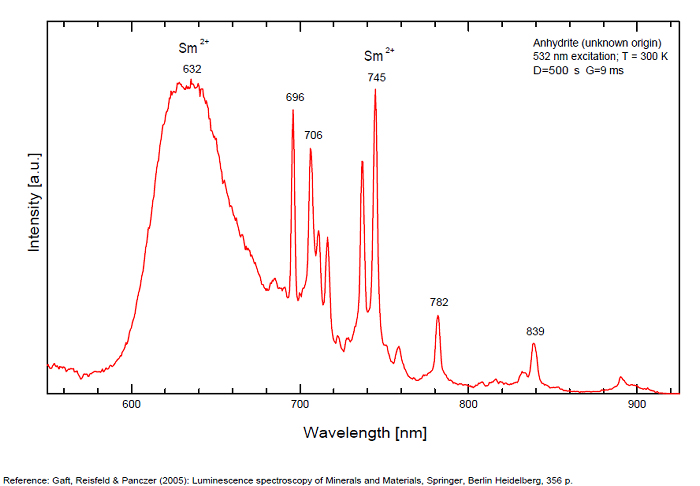
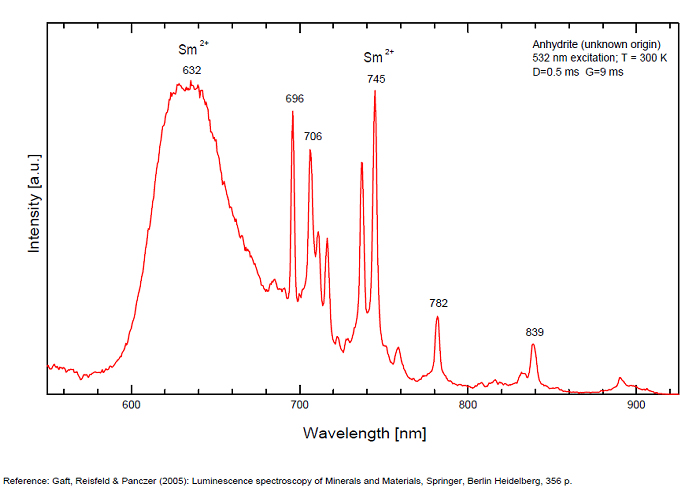
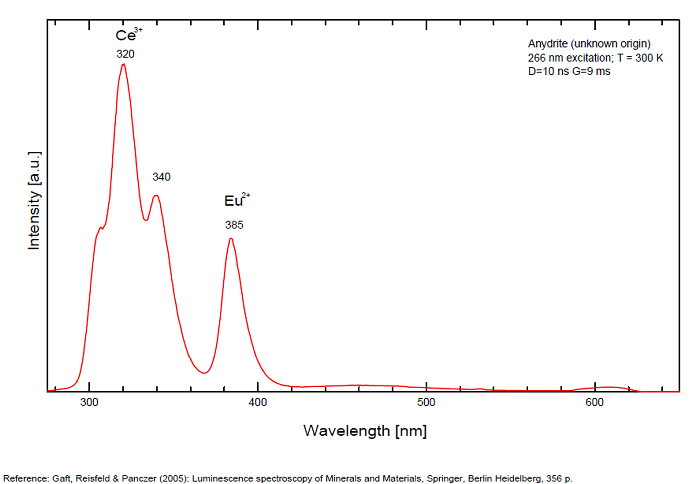
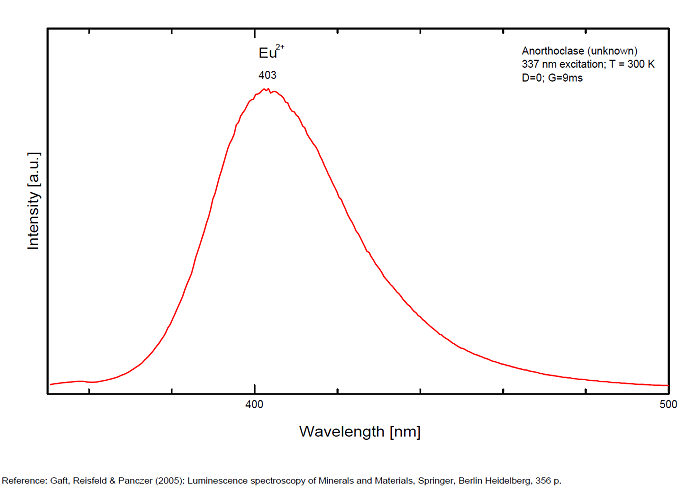
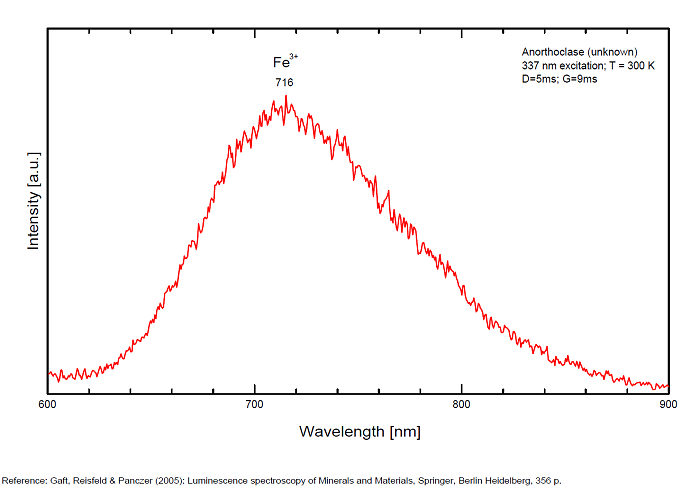
ANORTHOCLASE(Na,K)AlSi3O8
Activator(s):
Fe3+,
Other activators:
Cr3+, Eu2+, Ce3+, Eu3+,
Peaks in the spectrum (nm):
Fe3+: broad bands peaking at 716nm (110nm half-width)
Cr3+(?): broad bands peaking at 785nm
Eu2+: broad band at 403nm (40nm half-width)
Ce3+: broad bands peaking at 335nm
Eu3+: 404nm
Comments on spectra and activators:
Also broad bands peaking at 335 (Ce3+), 404 nm (Eu3+) (Gaft)
ANTHOPHYLLITEXMg7Si8O22(OH)2
Activator(s):
Mn2+ ,
Peaks in the spectrum (nm):
band with a peak at 398nm, 513, 585nm Mn2+ repl. Mg2+ : band peaking at 651-655 nm
Comments on spectra and activators:
The photoluminescence spectra, optical excitation spectra and PL decay curves of anthophyllite from Canada were obtained at 300 and 10 K by Aierken Sidike and Al.. The MnO content in the sample, determined using an electron probe microanalyzer, was high at 5.77 wt%. In the photoluminescence spectra obtained under 410nm excitation, bright red bands with peaks at 651 and 659nm were observed at 300 and 10 K, respectively. The origin of the red luminescence was ascribed to Mn2+ in anthophyllite from the analysis of the excitation spectra and photoluminescence decay times of 6,1–6,6 ms. In the PL spectra obtained under 240nm excitation at 300 K, a small violet band with a peak at 398 nm was observed. On the violet band at 10 K, a vibronic structure was observed. The origin of the violet luminescence was attributed to a minor impurity in anthophyllite.
(Source: Photoluminescence properties of anthophyllite, Aierken Sidike, Nuerrula Jilili, S. Kobayashi, K. Atobe and Nobuhiko Yamashita, Physics and Chemistry of Minerals, vol37 , 2009)
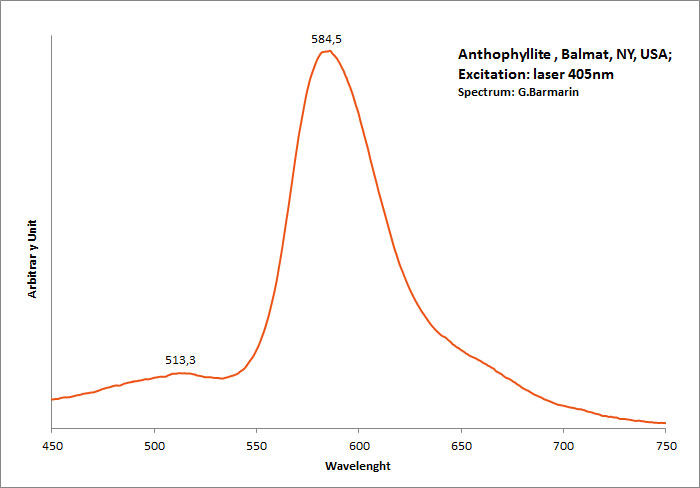
Excitation: laser 405nm. Col. G. Barmarin; Spectre: G. Barmarin
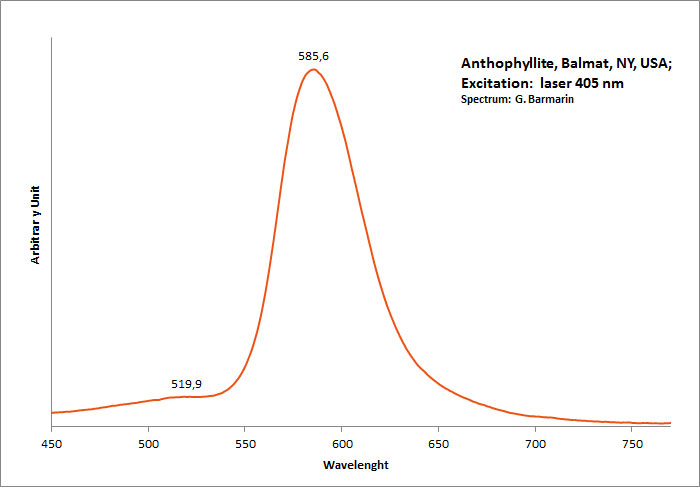
ARAGONITECaCO3
Activator(s):
Mn2+ ,
Other activators:
(UO2)2+ (ion Uranyle) en impureté, ST (Singlet-triplet)-Matière organique en impureté, Ce3+, Sm3+, Eu3+, Dy3+, Centres dûs aux effets des radiations,
Peaks in the spectrum (nm):
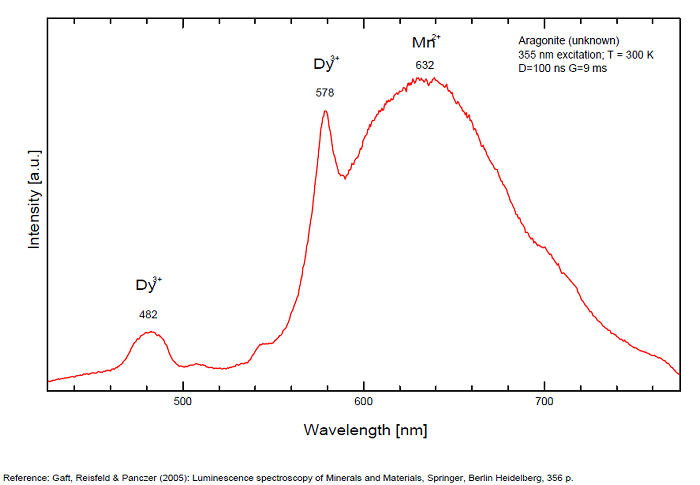
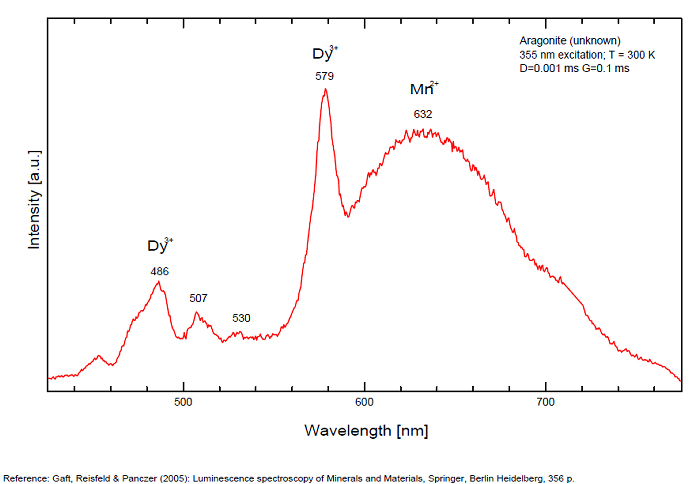
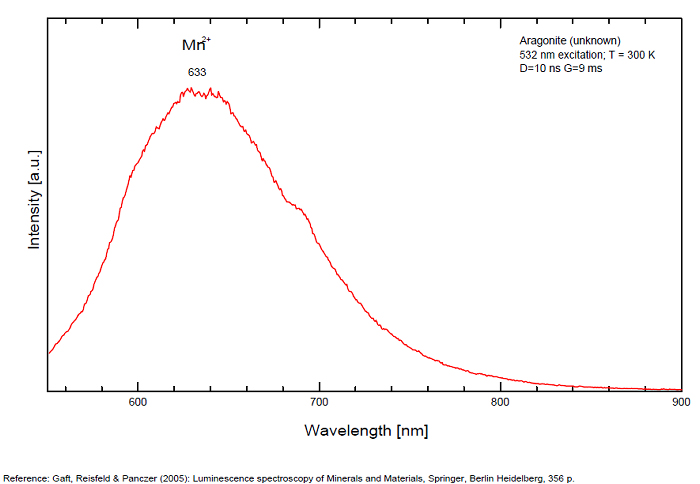
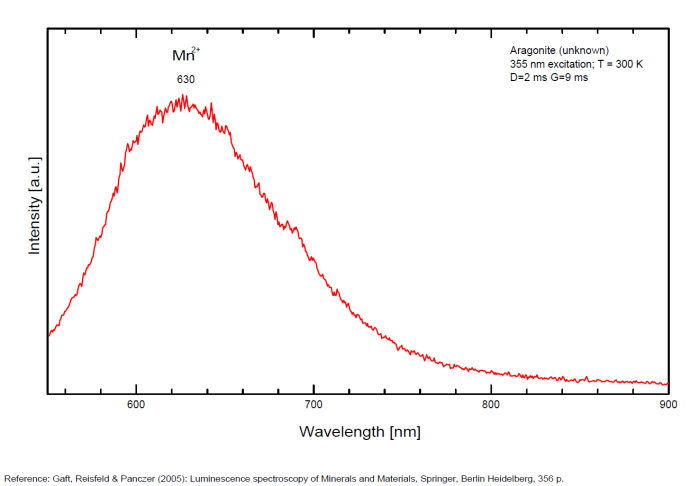
Sm3+ repl. Ca2+ : 603, 640nm Mn2+ : broad band at 630nm (120nm half-width) Dy3+ : 482, 486, 578, 579nm Radiation induced center : 580nm (very short decay time of approximately 20 ns)
Comments on spectra and activators:
Fluo green: due to U in traces; Under cw laser excitation at 532 and 780nm several bands and narrow lines have been found in visible and IR parts of the spectrum. Evidently they may be connected with another type of Mn2+, trivalent REE, such as Sm3+, Eu3+ and different types of Nd3+ centers. The origin of the broad band peaking at approximately 700 nm needs further study. The frequent occurrence of slight amounts of Strontium in aragonite was known during the time of Becquerel and he ascribed the luminescence to the presence of strontium. Later, Nichols confirmed the idea. Hence the name strontioaragonite for some specimen of very bright red fluorescing aragonite. Actually, strontium is not considered anymore as the activator responsable for the red fluorescence of aragonite and Mn and REE are considered as the principal activators of this red fluorescence.
Activators: Mn2+, Sm3+, Dy3+ (Gaft)
The broad luminescence band peaking at 580 nm with very short decay time of approximately 20 ns, could by analogy with Terlingua-type calcite may be preliminarily ascribed to radiation induced luminescence center. (Gaft)
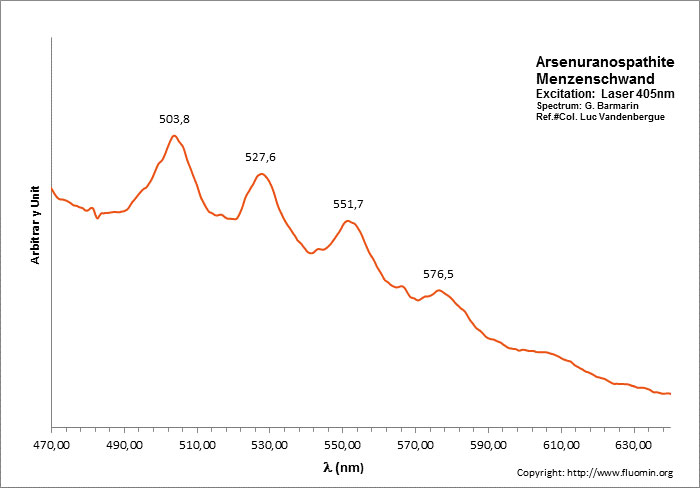
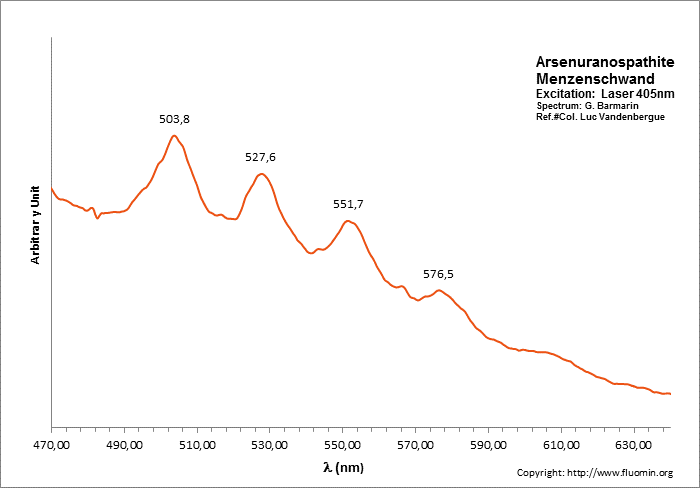
ARSENURANOSPATHITEHAl(UO2)4(AsO4)4 40H2O
Activator(s):
(UO2)2+ (ion Uranyle) intrinsèque ,
Peaks in the spectrum (nm):
(UO2)2+ : 504, 528, 552, 576nm
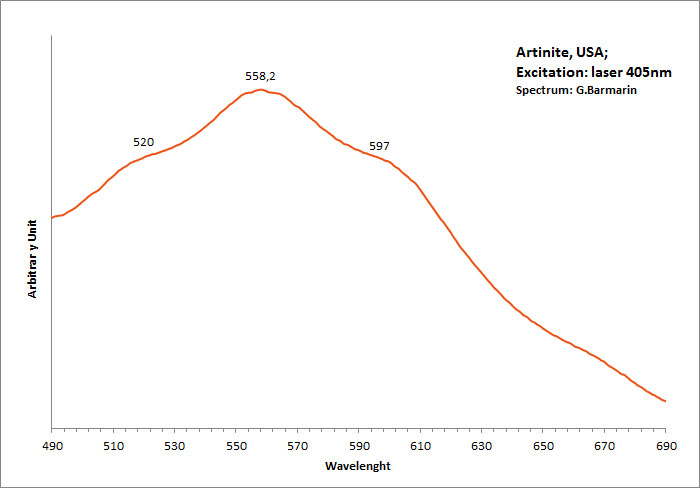
ARTINITEMg2(CO3)(OH)2 3H2O
Activator(s):
,
Peaks in the spectrum (nm):
Broad band peaking at 558.2nm with shoulders at 520 and 597nm
AUSTINITECaZn(AsO4)(OH)
Activator(s):
(UO2)2+ (ion Uranyle) en impureté,
Peaks in the spectrum (nm):
UO22+ : 502, 521, 545nm
Comments on spectra and activators:
Typical spectrum from Uranyl impurities.
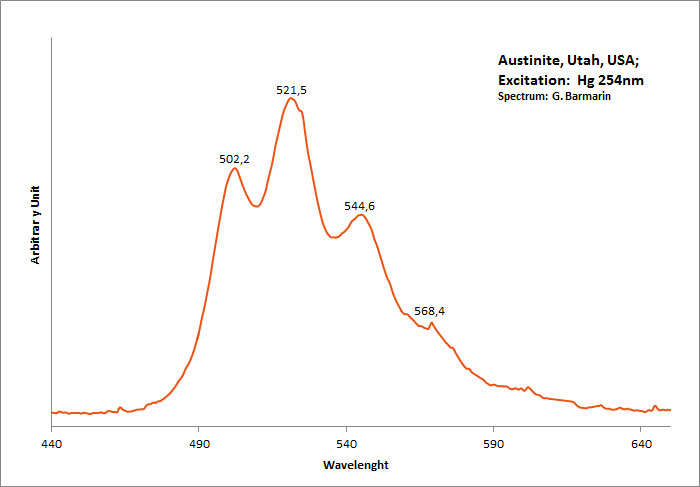
Excitation: UVSW 254nm (Superbright). Col. G. Barmarin; Spectre: G. Barmarin
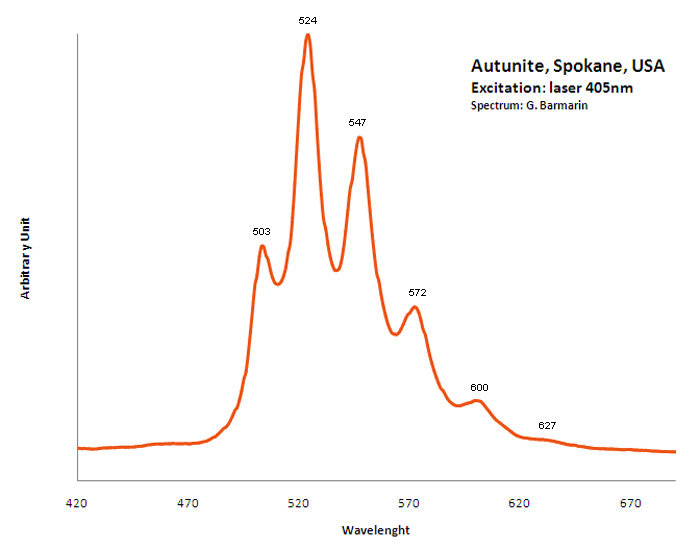
AUTUNITECa(UO2)2(PO4)2 11H2O
Activator(s):
(UO2)2+ (ion Uranyle) intrinsèque ,
Peaks in the spectrum (nm):
488.6, 504, 524.2, 548, 573.9, 602.4 - (488), 503, 524,(547), 550,(572), 600, (627)
Comments on spectra and activators:
Pics pour la meta-autunite: 488, 501.6, 523.7, 547.2, 573.3, 600.2 ; Lifetime: 60μs ;
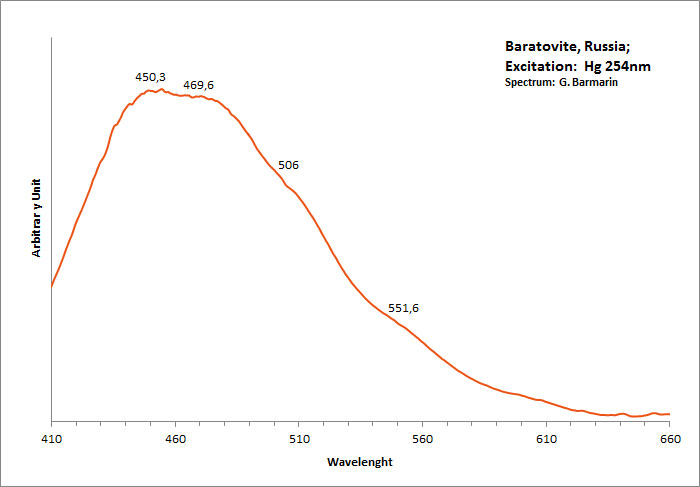
BARATOVITEKCa7(Ti,Zr)2Li3Si12O36(OH,F)2
Activator(s):
Ce3+,
Other activators:
Sm3+, Dy3+,
Peaks in the spectrum (nm):
Ce3+ repl. Ca2+ : 390 Sm3+ repl. Ca2+ : 600, 645 Dy3+ repl. Ca2+ : 490, 580
Comments on spectra and activators:
https://www.researchgate.net/publication/226256619_Photoluminescence_of_baratovite_and_katayamalite )"> Large band centered at 406nm? (see https://www.researchgate.net/publication/226256619_Photoluminescence_of_baratovite_and_katayamalite )
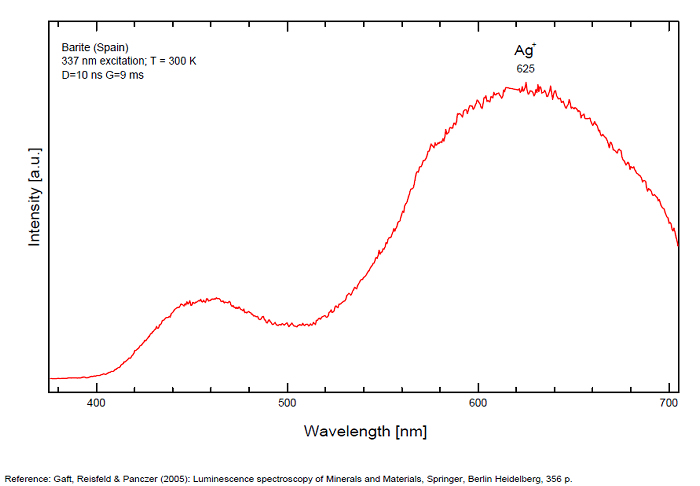
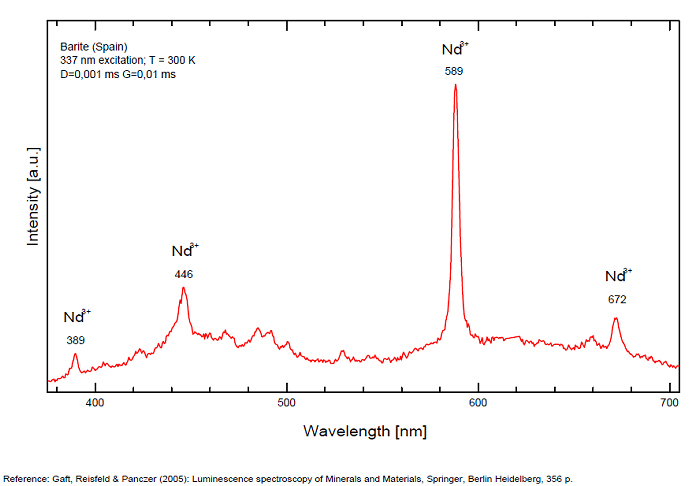
BARYTEBaSO4
Activator(s):
(UO2)2+ (ion Uranyle) en impureté,
Other activators:
Pb2+, S2-, ST (Singlet-triplet)-Matière organique en impureté, Cu+, Eu2+, Ce3+, Tb3+, Ag+, Nd3+, Bi2+, Bi3+,
Peaks in the spectrum (nm):
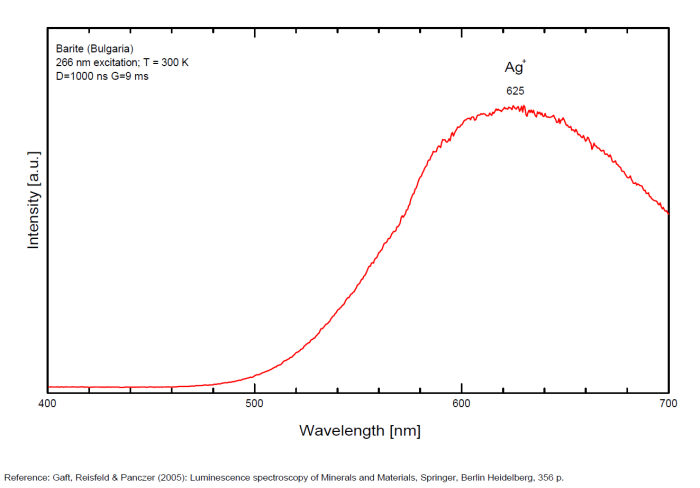
Bi3+ : 426-430nm band 80nm half-width ( τ = 750 microsecondes) Bi2+ replacing Ba2+ : narrow band 625nm ( τ = 5 microsecondes) Ag+ : very broad band (150 nm half-width) peaking at 635nm ( τ = 225 microsecondes) Ce3+ : 302, 305, 330, 360nm Nd3+ : 389, 446, 589, 672nm Eu3+ : 592, 620, 701nm Tb3+ : 488, 544nm UO22+ : 501, 522, 544, 568 Eu2+ : 375, 613, 615, 697nm Pb : blue part of the spectrum
Comments on spectra and activators:
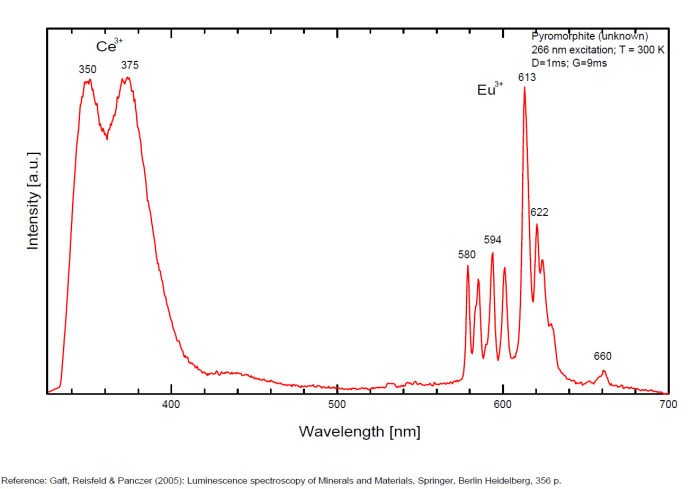
It is known that the luminescent centers Eu2+ (1.24–1.40 Å), Ce3+ (0.88–1.02 Å), Nd3+ (0.99–1.15 Å), Tb3+ (0.89–1.09 Å), Ag+ (1.13–1.26 Å), Sn2+ (0.93 Å) substitute for Ba2+ (1.35–1.44 Å) or Sr2+ (1.10–1.27 Å), which are in 12-fold coordination in the barite structure (all ionic radii are for the 6-coordination form) (GOROBETS, 2002; GAFT et al., 2005); A possible accommodation for the established in this study Eu3+ (0.97–1.13 Å) is also isomorphic substitution for Ba2+ or Sr2+ Activators: (UO2)2+ UO22+ can be determined from the vibrational structure and the long decay time of the luminescence band. Two different types of uranyl could be detected in Baryte: thin films of uranyl mineral considered to be a result of chemical adsorption (GOROBETS, 2002) and a solid solution of uranyl ion in barite crystal. Activators: Eu2+ Eu2+ can be determined from the spectral position, the half-width and the characteristic decay time of the luminescence band. Mn2+ and Ag+ can be determined by comparing luminescence bands spectral parameters to those of synthesized BaSO4−Mn and BaSO4−Ag. The luminescence spectrum (excitation of 266 nm, without delay, broad gate of 9 ms) of the studied endogenous barite contains 2 relatively narrow ultraviolet bands: one peaking at 306 nm and the other at 375 nm. The first band has a very short decay time and disappears after D = 50–100 ns. Such a combination of spectrum and decay time parameters is evidence that the luminescence is connected with Ce3+. The emission of Ce3+ corresponds to transitions between 5d1 and 4f1 electronic configurations. The second band has a longer decay time of approximately 1 μs and belongs to Eu2+ (GAFT et al., 2005). The Emission spectra of Eu2+ result from electronic transitions between 4f7 and 4f65d1 electronic configurations. After a delay of several μs, the Eu2+ emission becomes much weaker and very weak narrow lines appear, peaking at 488, 544 and 615 nm ). These lines are connected with trivalent rare-earth elements, which are characterized by relatively long decay times of hundreds of μs: the first 2 lines certainly belong to Tb3+ and the last one is principally typical for Eu3+ (GAFT et al., 2005). While Eu2+ luminescence is common, Eu3+ emission is here detected for the first time in barite. Under the excitation of 532 nm, a luminescence line at 615 nm dominates the spectrum, accompanied by several lines near 700 nm. Such behavior confirms that Eu3+ is responsible for these luminescence lines. The emission of Eu3+ corresponds to f–f transitions – from the excited 5D0 level to the 7Fj (j=0, 1, 2, 3, 4, 5, 6) levels of the 4f6 configuration. (Spectroscopic study of barite from the Kremikovtsi deposit (Bulgaria) with implication for its origin, MAYA DIMOVA, GERARD PANCZER & MICHAEL GAFT, ANNALES GÉOLOGIQUES DE LA PÉNINSULE BALKANIQUE, Vol67, 2006 See link below)
Ag+ (large pic 635 nm), Bi2+ in Ba2+ site (narrow band 625 nm), Bi3+ (426 nm), Eu2+ (375), Ce3+ (302, 330, 360nm), Nd3+ (446, 589nm) (Gaft)
Fe3+ or Mn4+ can be determined from the spectral-kinetic parameters of the luminescence bands.( After M. Gaft and I. Rudenkova, Laser-induced luminescence of barite after thermal treatment, Journal of Thermal Analysis and Calorimetry vol42, 1994)
In natural barite the narrow orange band with λmax=625 nm, Δ=40 nm and τ=5 μs is connected with Bi2+ center.
The narrow violet band with λmax=625 nm, Δ=35 nm and τ=1.7 ms is connected with Bi3+ center.
The broad red band with λmax=635 nm, Δ=140 nm and τ=270 μs is connected with Ag+ center. The emission bands of Ag+ result from d9s–d10 transitions
The broad red band with λmax=750 nm, Δ=110 nm and τ=350 μs is connected with Cu+ center.(The luminescence of Bi, Ag and Cu in natural and synthetic barite , M. Gaft, R. Reisfeldb, G. Panczer, G. Boulonc, T. Saraidarovb and S. Erlishd, Optical Materials, vol16, 2001)
The different emission spectra at excitations at 266 and 532 nm suggest at least 2 structural positions for Eu3+ in the barite crystal lattice.
BENITOITEBaTiSi3O9
Activator(s):
TiO6,
Other activators:
Cr3+, Fe3+, Ti3+, Ti4+, Cu+, Mn4+, Ti - O,
Peaks in the spectrum (nm):
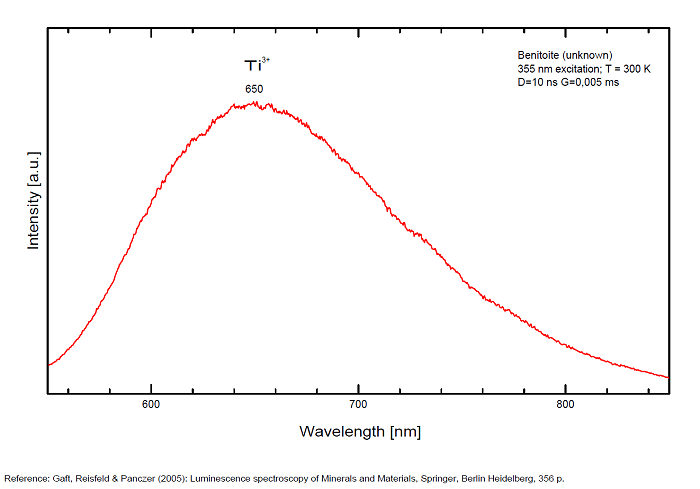
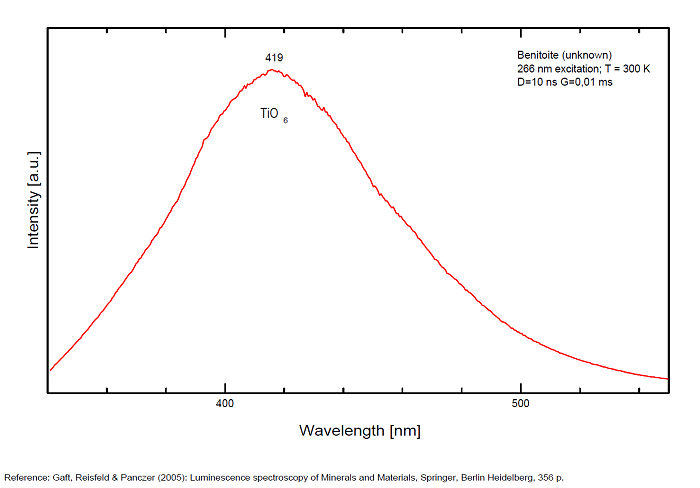
TiO6 : broad band at 419nm (due to the transition of 3T1 → 1A1 in the TiO6 octahedra)
Ti3+ : broad band around 650-660nm with half-width of +/-135nm
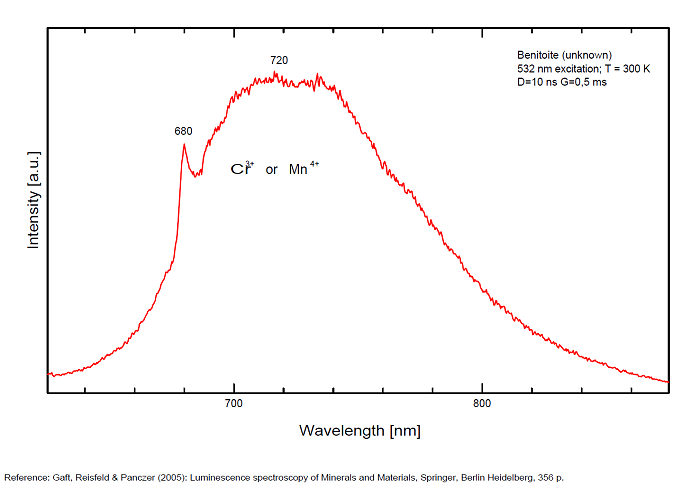
Cr3+ : peak at 680nm
Mn4+ : large band at 720nm
Cu+? replacing Ba2+ and Ti3+? (exc 337nm:(Gorobets)): 720nm
450nm (exc 254nm)
Comments on spectra and activators:
Robbins cites Fe as activator for the red color and Ti for the blue; recent spectrographic studies give new data: Decay time shortening and thermal quenching are connected with nonradiative decay within the TiO6 luminescence center, while energy migration does not take place at least up to room temperature.
There are three broadbands:
- one around 419 nm (blue band) is connected with intrinsic luminescence centers, namely isolated TiO6 octahedra,
- one at 650 nm (Yellow band) for Ti3+ ,
- one at 720 (half-width 125nm) for Mn4+
and a narrow line at 680 for Cr3+.(Gaft)
Manganese participation is supported by chemical analysis of benitoite (0.03 to 0.11%).
The metastable level 3T1u is the emitting level at low temperatures with a long decay time of 1.1 ms. At higher temperatures an energy level with higher radiation probability must be involved in the emission process, and this level is situated at 0.06 eV higher than the lowest level. These two levels may be connected with 3T1u level splitting or with closely spaced 3T1u and 3T2u levels.
(Source: M. Gaft, L. Nagli, G. Waychunas and D. Weiss, The nature of blue luminescence from natural benitoite, Physics and Chemistry of Minerals, vol31, 2004)
The red emission of benitoite consists of two individual bands and one line and suggest that the activators of luminescence in benitoite system are Ti3+ and a d3 element, namely Cr3+ or Mn4+.
(Source: M. Gaft, L. Nagli, G. Waychunas and G. Panczer, The nature of red luminescence of natural benitoite, Mineralogy and Petrology, Vol85, 2005)
BERGENITECa2Ba4[(UO2)3O2(PO4)2]3 16H2O
Activator(s):
(UO2)2+ (ion Uranyle) intrinsèque ,
Peaks in the spectrum (nm):
504nm, 524nm, 540nm, 564nm, (592nm)
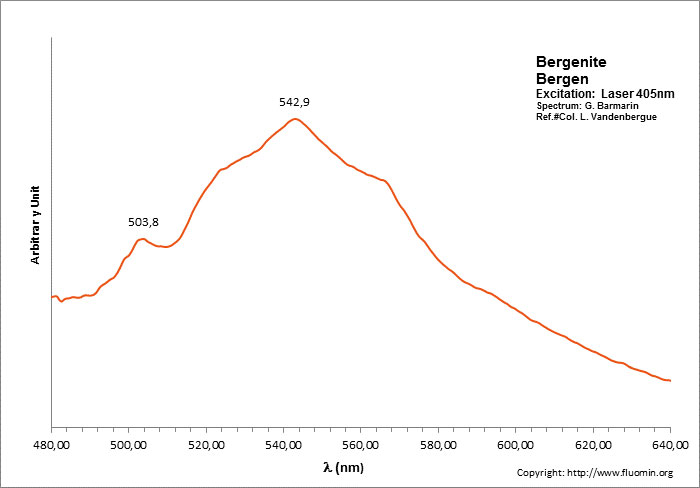
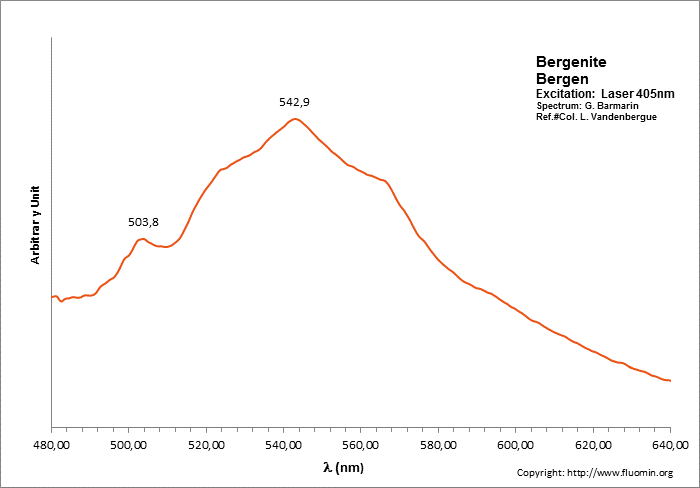
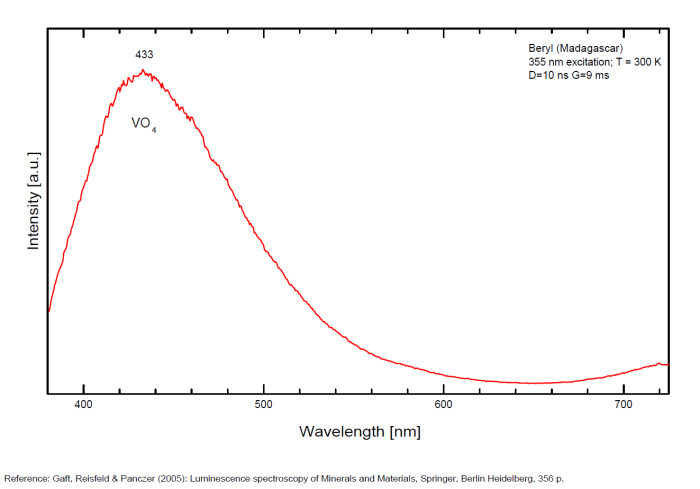
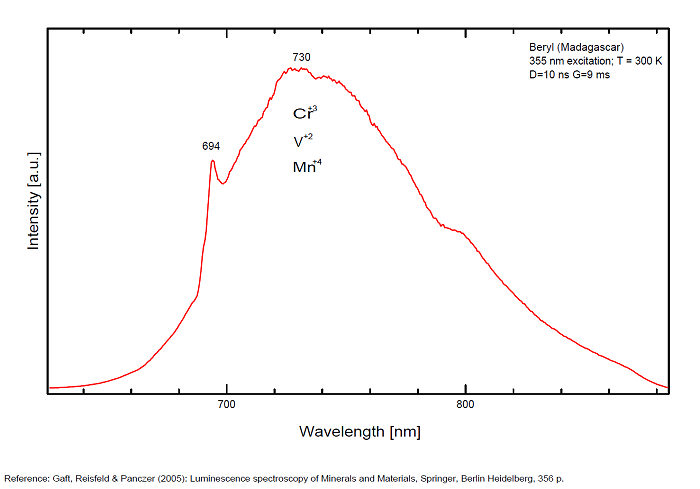
BERYLBe3Al2Si6O18
Activator(s):
Cr3+,
Other activators:
Fe3+, Mn4+, V2+, VO43-,
Peaks in the spectrum (nm):
(VO4)3- : Broad band peaking at 433 nm Mn2+ tetrahedral : Broad band peaking at 480nm Mn2+ octaedral : Broad band peaking at 570nm Fe3+ or V2+ or Mn4+ : Broad band peaking at 720 - 745 nm (140nm half-width) Cr3+ or V2+ : Lines at 692 and 694nm
Comments on spectra and activators:
Band peaking at 730 nm associated with Mn4+ (or Mn3+)
Broad band peaking at 480 (Mn2+ in tetrahedral) and 570 (Mn2+ in octaedral coordination).
Broad band peaking at 720 - 745 nm (Fe3+ or V2+ or Mn4+).
Lines at 692 and 694 (Cr3+).
Broad band peaking at 433 nm (VO4).
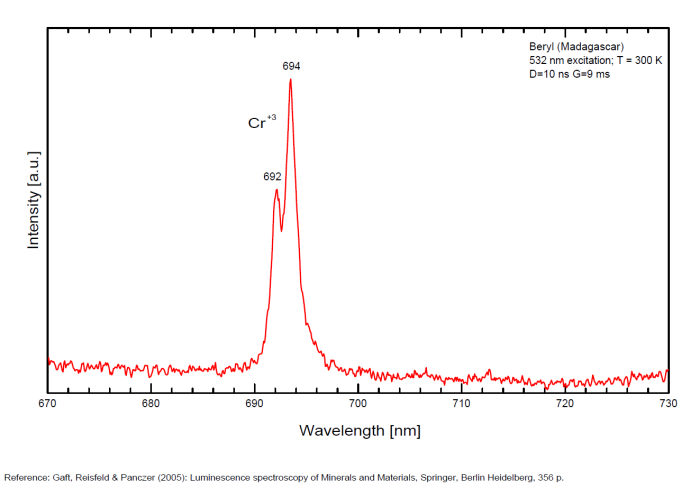
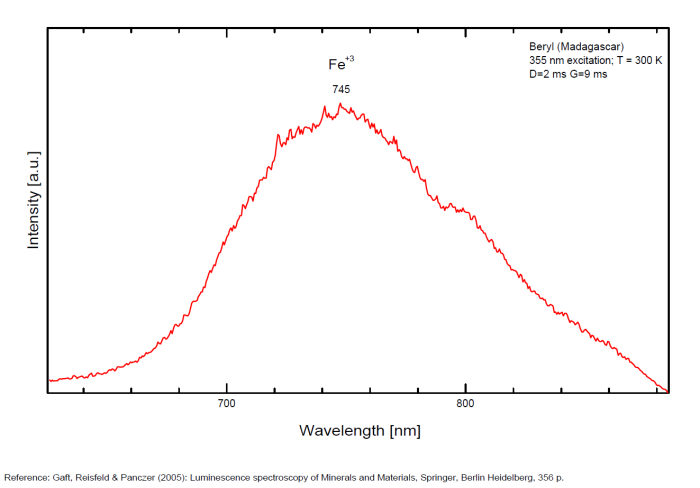
BILLIETITEBa(UO2)6O4(OH6)4-8H2O
Activator(s):
(UO2)2+ (ion Uranyle) intrinsèque ,
Peaks in the spectrum (nm):
Large band peaking at 536nm ?
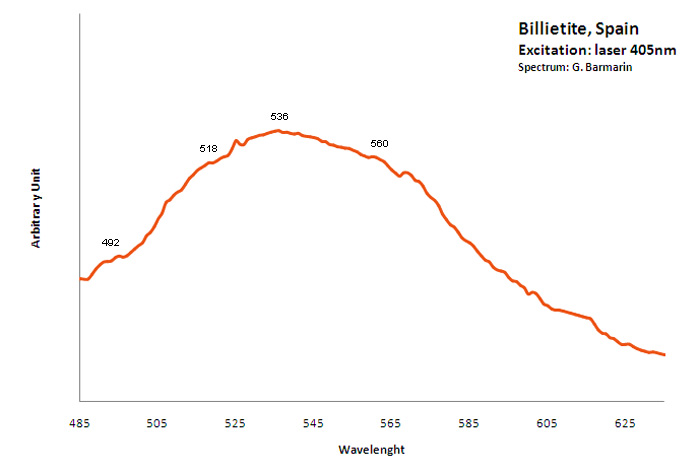
BITYITECaLiAl2BeAlSi2O10(OH)2
Activator(s):
(UO2)2+ (ion Uranyle) en impureté,
Peaks in the spectrum (nm):
(UO2)2+ : broad band peaking at 517.7 and 556nm;
The specified directory does not exist.
BOEHMITEAlO(OH)
Activator(s):
Cr3+,
Peaks in the spectrum (nm):
Cr3+ : 692, 700, 711nm Cathodoluminescence: orange (Khininy);
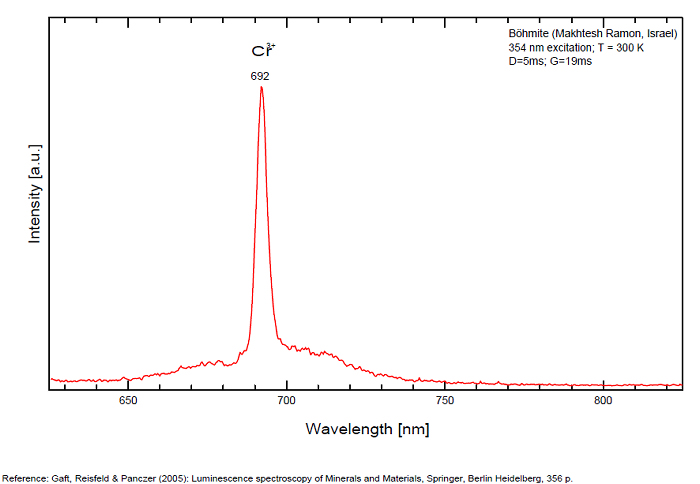
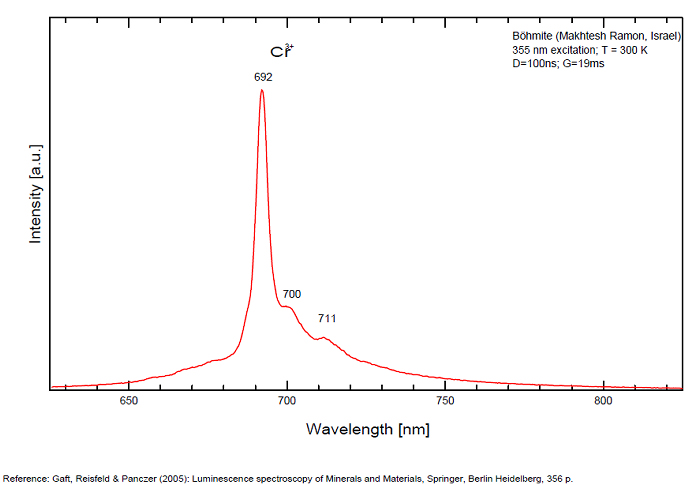
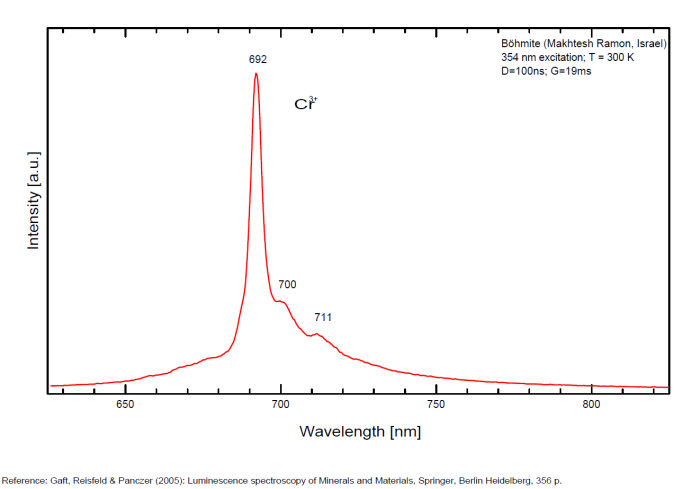
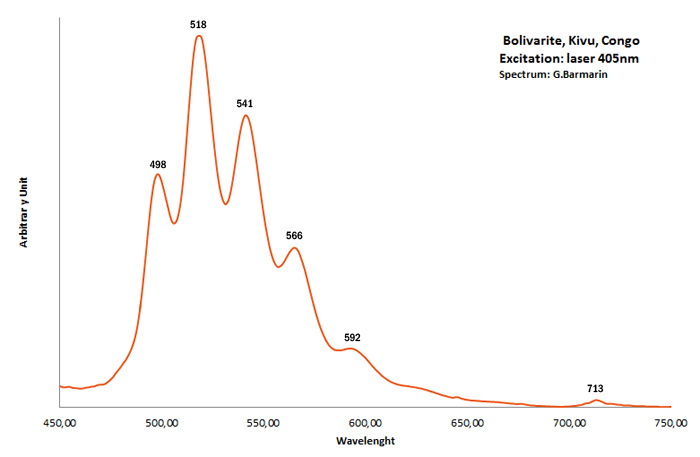
BOLIVARITEAl2(PO4)(OH)3 4H2O
Activator(s):
(UO2)2+ (ion Uranyle) en impureté,
Peaks in the spectrum (nm):
UO22+ : 498, 518, 541, 566, 592nm 713nm
BOLTWOODITE(K,Na)(UO2)(SiO3OH) 1,5H2O
Activator(s):
(UO2)2+ (ion Uranyle) intrinsèque ,
Peaks in the spectrum (nm):
470, 500, 520, 545, (555), 565-570 large band around 578nm with events at 503, 521, 563 and 602nm 485.1, 501.5, 521.2, 543.0, 567.4, and 591.4 nm (Thuro Arnold and Nils Baumann)
Comments on spectra and activators:
Gorobets: 18850 cm-1, 18050 cm-1; fluorescence lifetimes of 382 and 2130 ns (bi-exponential)
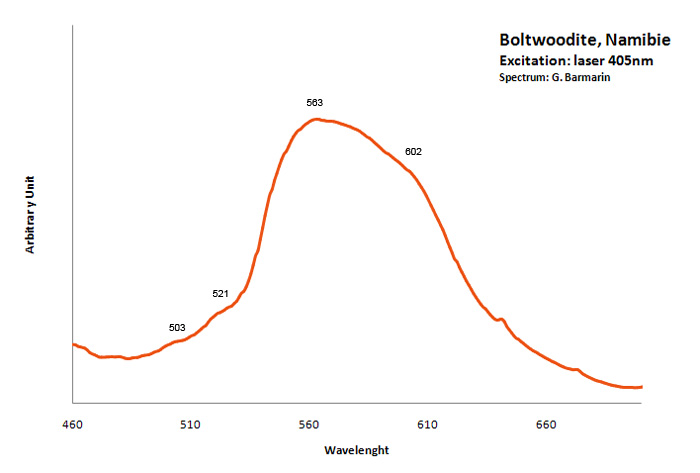
CALCITECaCO3
Activator(s):
Mn2+ ,
Other activators:
Pb2+, ST (Singlet-triplet)-Matière organique en impureté, Ce3+, Sm3+, Eu3+, Dy3+, Tb3+, Nd3+, Centres dûs aux effets des radiations, Tm3+, PO2* , CO3-* ,
Peaks in the spectrum (nm):
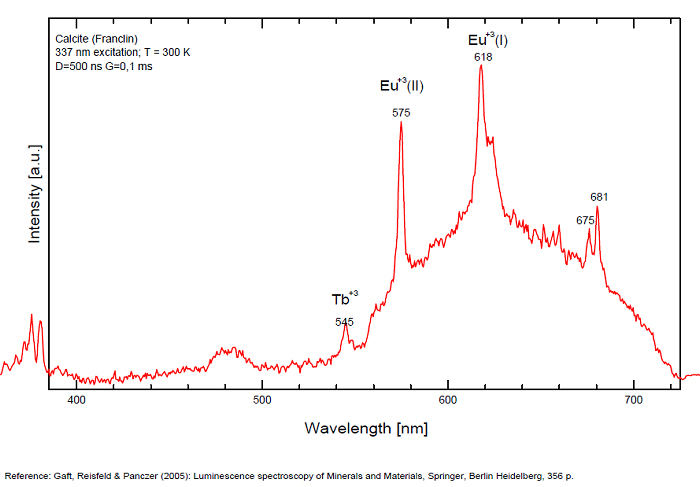
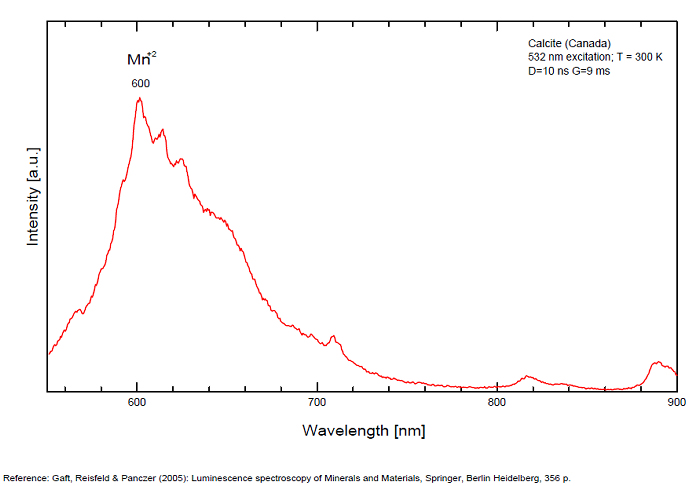
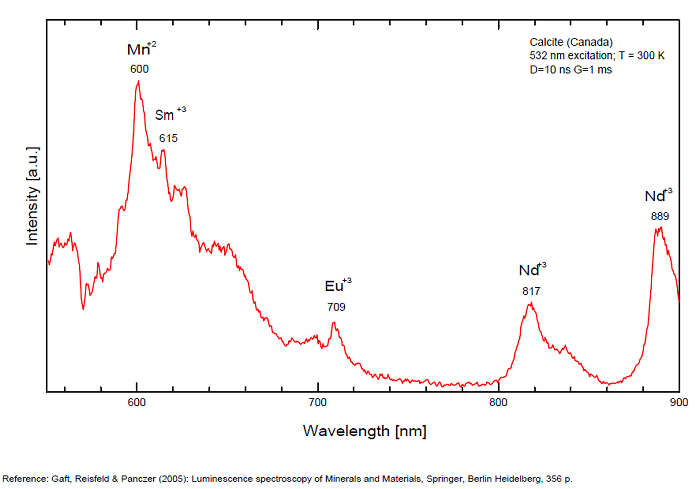
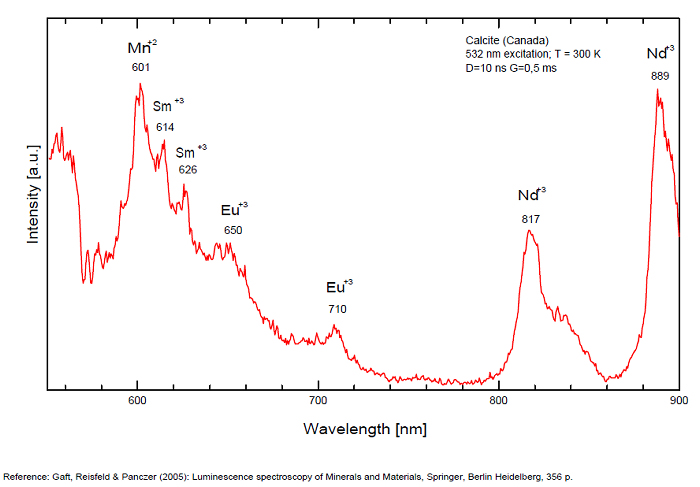
Pb2+ repl. Ca2+ : band at 320-325nm (60nm half-width) Ce3+I : 350-355, 380nm (60nm half-width) Ce3+II : 400nm Mn2+II : 580 - 620nm ( band with 100nm half-width) Mn2+I repl. Ca2+ : 610 - 630nm (band) ST : 400-500nm and 450-550nm [CO3-]* intr. : 420nm (radiation induced CO33- ) [PO2]*? repl. [CO3] : 570nm Nd3+ : 817, 889nm Sm3+ : 614, 615, 626nm Dy3+ : 483, 577nm Tb3+ : 544, 545nm Eu3+I : 591, 618, 696, 709nm Eu3+II : 575nm Eu3+ : 592, 619nm Radiation induced centers : 415-420, 550nm (in pure Iceland spar)
Comments on spectra and activators:
In nature, RE and Mn2+ can substitute for Ca, becoming luminescence centers. Most calcite luminescence is attributed to Mn2+, while Mn2+ hides rare earth emission, and so far their luminescence in calcite is rather poorly characterized. Activator: Mn2+ substituting to Ca2+ (large peak around 610 - 630 nm); also Pb2+ replacing Ca2+ with a coordination number of 6 producing 312-325nm (Tarashchan) and radiation-induced (CO3)3- 403 nm (Kasyanenko); also RRE: Ce3+, Sm3+, Dy3+, Eu3+, Tb3+, Tm3+, Nd3+ (Gaft) Both luminescence centers are thermally unstable with the blue emission disappearing after heating at 500 °C, and the orange emission disappearing after heating at different temperatures starting from 230 °C, although sometimes it is stable up to 500 °C in different samples. Stokes (1852) was unable to observe the fluorescence of calcite. In 1859, Becquerel found that fluorescent calcite contained up to 2,7% Mn. In 1867 he studied calcite with his new phosphoroscope measuring a 0,5s for phosphorescence. Lommel (1884) studied calcite using sun as UV source and in 1909, Pochettino employed cathode ray to study the fluorescence of calcite. Sohncke (1896) while investigating polarized fluorescence found calcite from Iceland showing a marked fluorescence. Nichols, Howes and Wilbur were of opinion that Mn traces cause the luminescence of calcite and demonstrated this with certainty in later investigations. Tanaka working in the Cornell Laboratory (1924) also concluded that the chief activator in calcite, crystalline limestone and dolomite was manganese. Pringsheim (1928) found also Mn as the most important activator in calcite. In 1939, G. Fonda made the synthesis of fluorescent calcite by precipitation of calcium carbonate in presence of a manganese salt. But the product is not exactly as calcite in nature. Another technic used to obtain fluorescent calcite is to firing a small amount of manganese salt (about 0,005%) in calcium carbonate for about one hour at 1000° (in an atmosphere of carbon dioxide). Another successful synthesis of luminescent calcite, closely resembling the salmon red fluorescing calcite of Franklin is achieved by precipitating the carbonate from a mixture of calcium and manganese chlorides by ammonium carbonate under special conditions. The optimum concentration for the dilution of the chlorides is 1Mn:30Ca. Dilute solutions containing 1,8% CaCl2.6H2O and 1,5%(NH4)2CO3.H2O, with 50% excess ammonium carbonate have been found best. The precipitation is carried out at about 70°C, followed by holding at room temperature for several weeks before filtering, boiling for 10 minutes gives similar results. With this mode of preparation, synthetic calcite has 30% the intensity of Franklin calcite. (text from Fluorescent Gems and Minerals by Jack De Ment, 1947) In 1943, De Ment identified the uranium luminescence spectrum of calcite from Deming, NM, USA under SW and the Terlingua, Texas, USA calcite. For Terlingua calcite, he found a strong band at 410-450nm (purple-blue), a bright green band at 450-545nm and a weak reddish band at 545-620nm. The blue long-lived phosphorescence have the same spectral bands as fluorescence. De Ment mentioned in 1947 the role of RRE as activator in calcite (Dy, Sm, Y, Ce) and of Thallium (?). Cathodoluminescence: light-blue, orange;
Natural calcite from Kuerle, Xinjiang, China, shows orange-red fluorescence when exposed to short-wave ultraviolet (UV) light (Hg 253.7nm).
The PL emission spectrum under 208 nm excitation consists of three bands: two UV bands at 325 and 355 nm and an orange-red band at 620 nm.
The three bands are ascribed to Pb2+, Ce3+ and Mn2+, respectively, as activators.
The Pb2+ excitation band is observed at 243 nm, and the Ce3+ excitation band at 295 nm.
The Pb2+ excitation band is also observed by monitoring the Ce3+ fluorescence, and the Pb2+ and Ce3+ excitation bands, in addition to six Mn2+ excitation bands,
These indicate that four types of the energy transfer can occur in calcite through the following processes:
(1) Pb2+ → Ce3+,
(2) Pb2+ → Mn2+,
(3) Ce3+ → Mn2+ and
(4) Pb2+ → Ce3+ → Mn2+.
(Source:Energy transfer among Pb, Ce and Mn in fluorescent calcite from Kuerle, Xinjiang, China,by Aierken Sidike, X.-M. Wang, Alifu Sawuti, H.-J. Zhu , I. Kusachi and N. Yamashita in Revue Physics and Chemistry of Minerals,Vol.33, Nov.2006, see link below)
The unusual luminescence of particular varieties of natural pink calcite (CaCO3) samples (Terlingua type) was studied by laser-induced time-resolved luminescence spectroscopy at different temperatures.
The luminescence is characterized by intense blue emission under shortwave UV lamp excitation with an extremely long decay time, accompanied by pink-orange luminescence under longwave UV excitation. This calcite contains negligible quantities of impurities, which may be potentially connected to such luminescence.
Investigation including optical absorption, natural thermostimulated luminescence (NTL) and Laser-Induced Breakdown Spectroscopy (LIBS) studies conduct to 2 luminescence centers: a narrow violet band, with max = 412 nm, FWHM= 45 nm, two decay components of 1 = 5 ns and 2 = 7.2 ms, accompanied by very long afterglow, and an orange emission band with max = 595 nm, FWHM= 90 nm, and = 5 ns.
Both centers have spectral-kinetic properties very unusual for mineral luminescence, which in combination with extremely low impurity concentrations prevent their identification with specific impurity related emission.
The most likely explanation of these observations may be the presence of radiation-induced luminescence centers. The long violet afterglow is evidently connected with trapped charge carrier liberation, with their subsequent migration through the valence band and ultimate recombination with a radiation-induced center responsible for the unusual violet luminescence.
(Source: The nature of unusual luminescence in natural calcite CaCO3, Michael Gaft, Lev Nagli, Gerard Panczer, Glenn Waychunas and Naomi Porat, American Mineralogist; January; v. 93; no. 1; p. 158-167, 2008 see link below)
Most common orange-red fluorescence is due to divalent manganese, with a co-activator to absorb the UV and transfer the energy to the manganese. Most common co-activator is probably lead. Cerium can act as a co-activator, suspected when the fluorescence is stronger with long-wave UV. Magnesium in the calcite will shift the spectrum a bit to the red side. Some calcites fluoresce closer to orange. Both trivalent dysprosium and trivalent samarium are said to be capable of producing orange fluorescence. Pink or yellow fluorescence with a greenish phosphorescence is suspected to be caused by organic activators. Green may be due to interstitial inclusion of mineral activated by uranium (often hyalite).
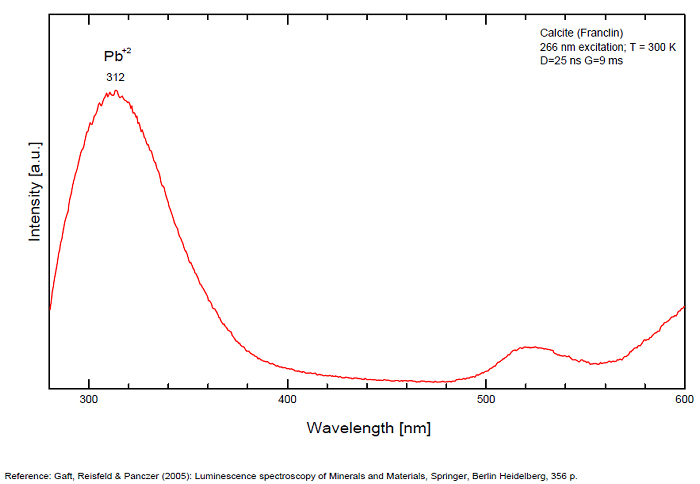
Spectrum: Michael Gaft, Petah Tikva, Israel. Plot: Institute of Mineralogy, University of Vienna, Austria, with permission of the authors.
Calcite, Franklin, USA, excitation 337nm
Spectrum: Michael Gaft, Petah Tikva, Israel. Plot: Institute of Mineralogy, University of Vienna, Austria, with permission of the authors.
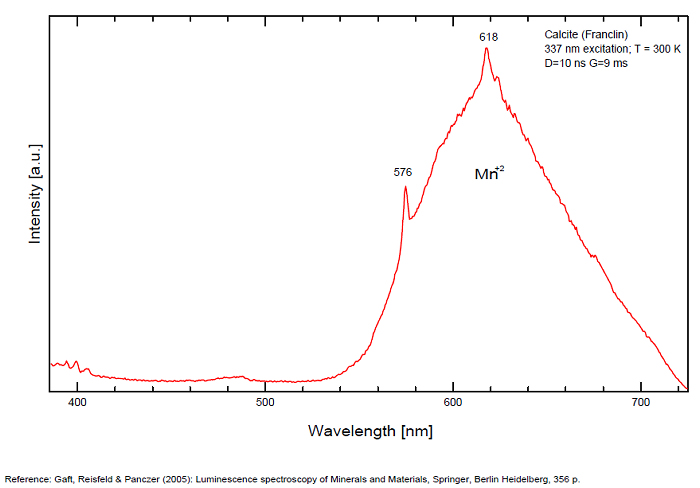
Spectrum: Michael Gaft, Petah Tikva, Israel. Plot: Institute of Mineralogy, University of Vienna, Austria, with permission of the authors.
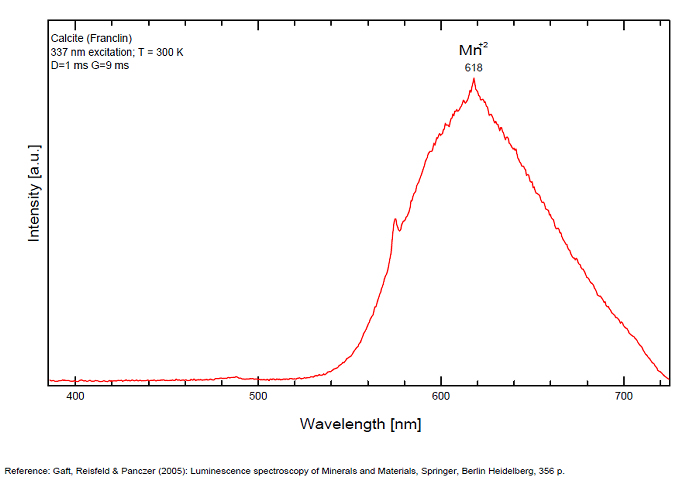
Spectrum: Michael Gaft, Petah Tikva, Israel. Plot: Institute of Mineralogy, University of Vienna, Austria, with permission of the authors.
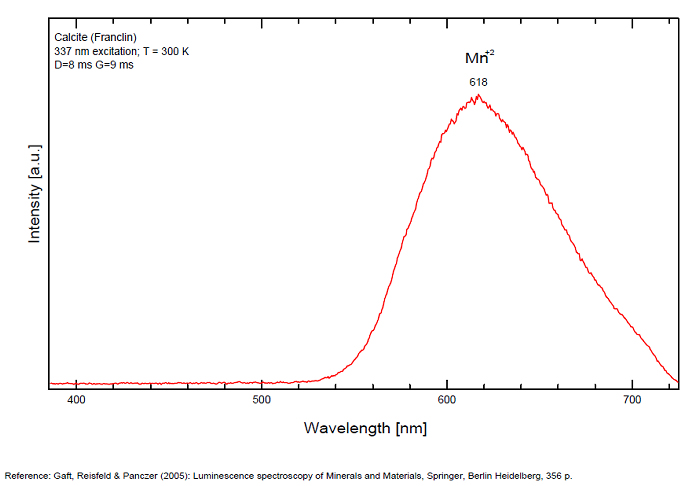
Spectrum: Michael Gaft, Petah Tikva, Israel. Plot: Institute of Mineralogy, University of Vienna, Austria, with permission of the authors.
Spectrum: Michael Gaft, Petah Tikva, Israel. Plot: Institute of Mineralogy, University of Vienna, Austria, with permission of the authors.
Spectrum: Michael Gaft, Petah Tikva, Israel. Plot: Institute of Mineralogy, University of Vienna, Austria, with permission of the authors.
Spectrum: Michael Gaft, Petah Tikva, Israel. Plot: Institute of Mineralogy, University of Vienna, Austria, with permission of the authors.
Spectrum: Michael Gaft, Petah Tikva, Israel. Plot: Institute of Mineralogy, University of Vienna, Austria, with permission of the authors.
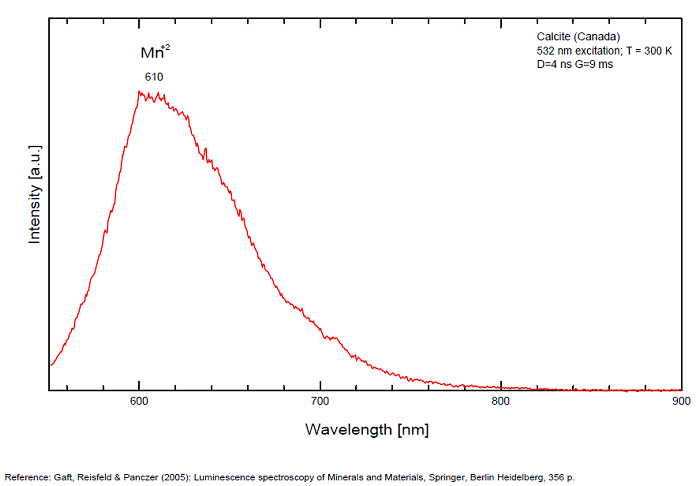
Spectrum: Michael Gaft, Petah Tikva, Israel. Plot: Institute of Mineralogy, University of Vienna, Austria, with permission of the authors.
Calcite, Franklin, USA, excitation 266nm
Spectrum: Michael Gaft, Petah Tikva, Israel. Plot: Institute of Mineralogy, University of Vienna, Austria, with permission of the authors.
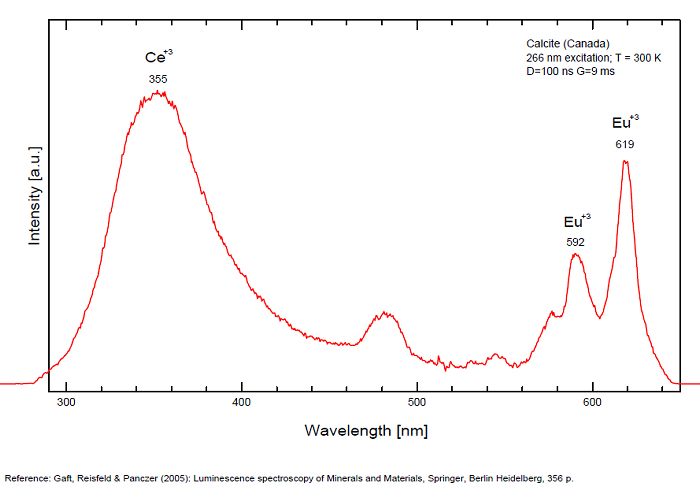
Spectrum: Michael Gaft, Petah Tikva, Israel. Plot: Institute of Mineralogy, University of Vienna, Austria, with permission of the authors.
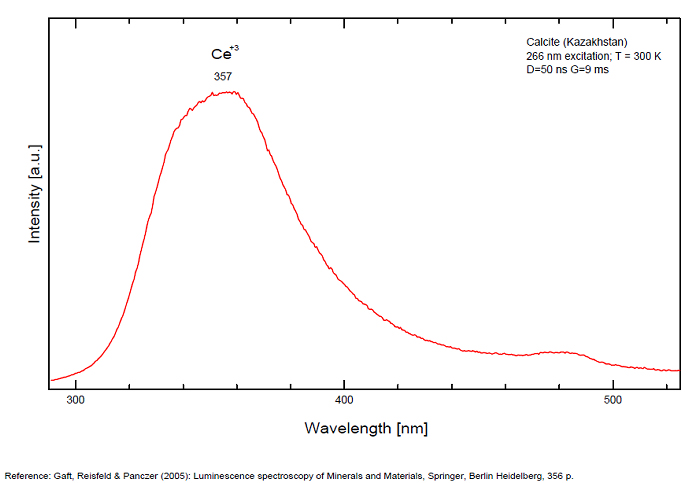
Spectrum: Michael Gaft, Petah Tikva, Israel. Plot: Institute of Mineralogy, University of Vienna, Austria, with permission of the authors.
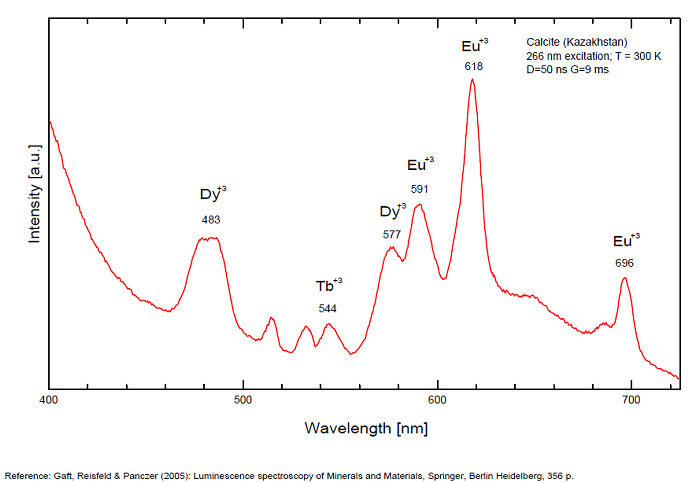
Spectrum: Michael Gaft, Petah Tikva, Israel. Plot: Institute of Mineralogy, University of Vienna, Austria, with permission of the authors.
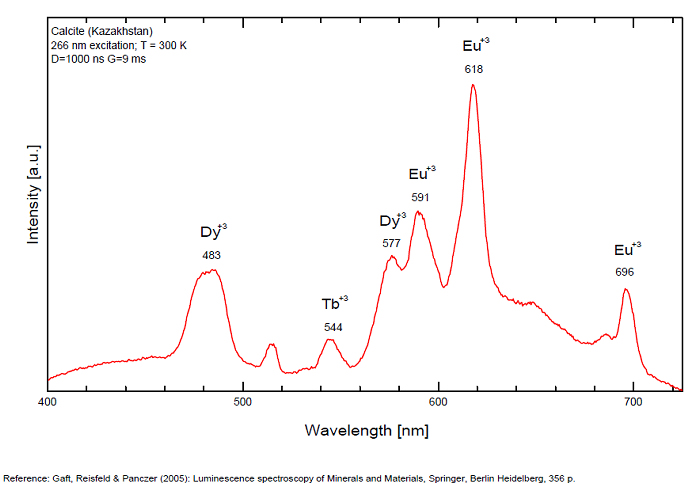
Spectrum: Michael Gaft, Petah Tikva, Israel. Plot: Institute of Mineralogy, University of Vienna, Austria, with permission of the authors.
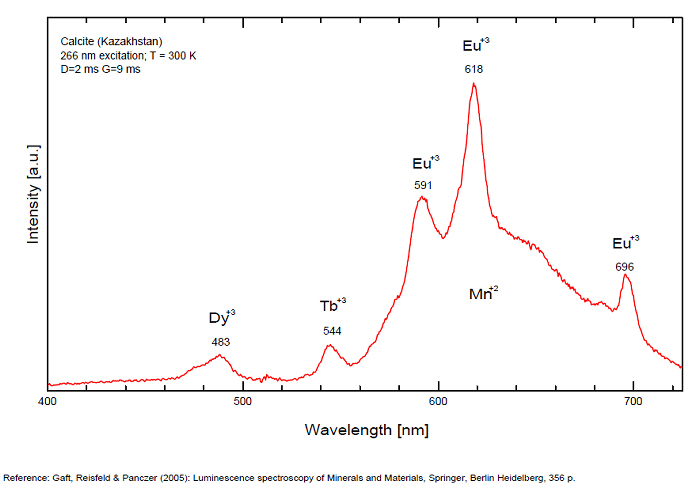
Spectrum: Michael Gaft, Petah Tikva, Israel. Plot: Institute of Mineralogy, University of Vienna, Austria, with permission of the authors.
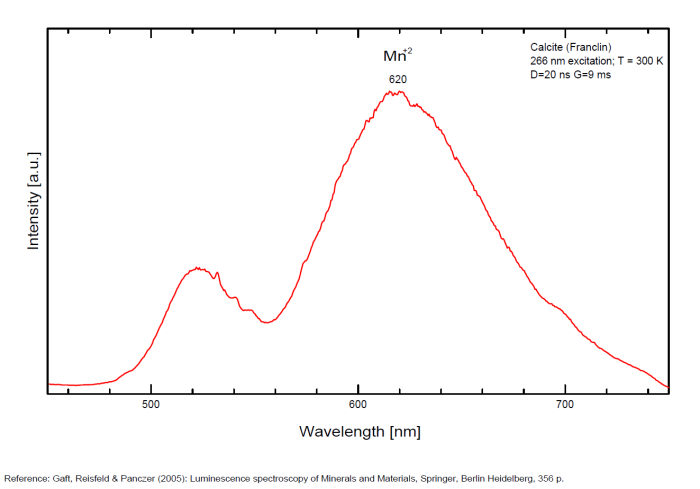
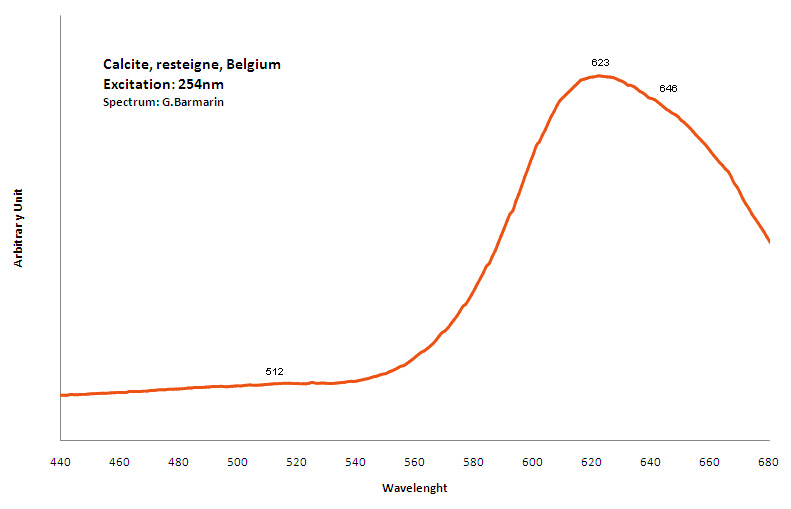
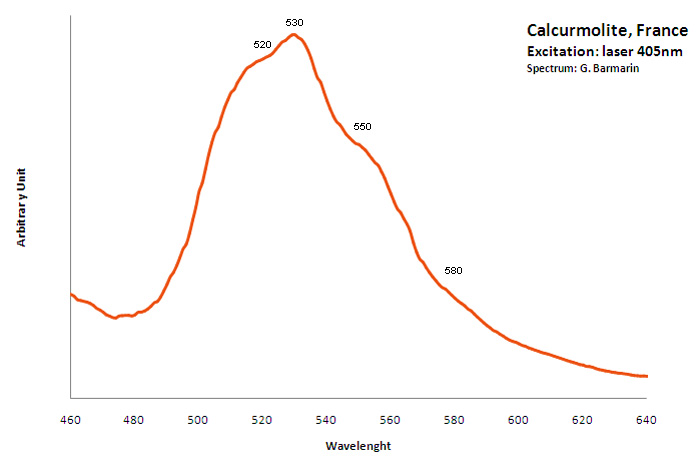
CALCURMOLITECa(UO2)3(MoO4)3(OH)2 11H2O
Activator(s):
(UO2)2+ (ion Uranyle) intrinsèque ,
Peaks in the spectrum (nm):
(UO2)2+ : (520nm), 530nm, (550nm), (580nm)
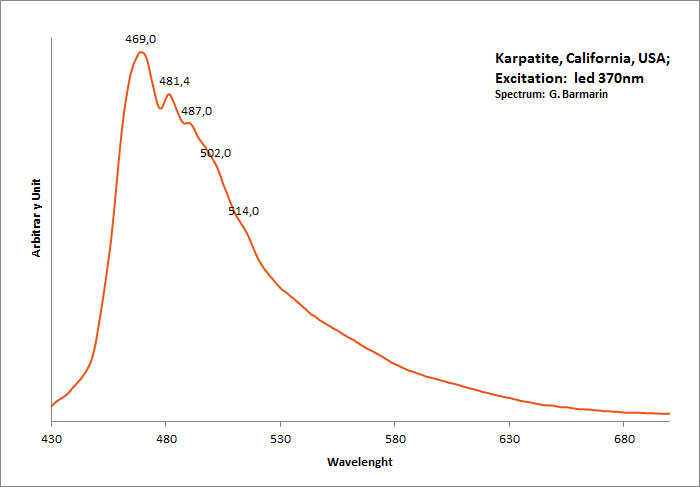
CARPATHITEC24H12
Activator(s):
Matière organique intrinsèque,
Peaks in the spectrum (nm):
462nm, (482), (493), (503), (515)
Comments on spectra and activators:
Blue luminescence due to the two linearly annelated benzene rings characteristic of aromatic compound (singlet-singlet electron transition within the benzene rings). Spectral lines (cm-1): 208, 192, 178, 164.
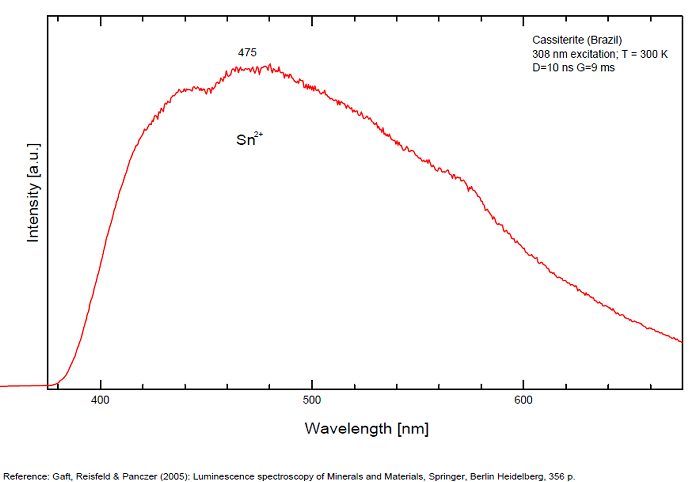
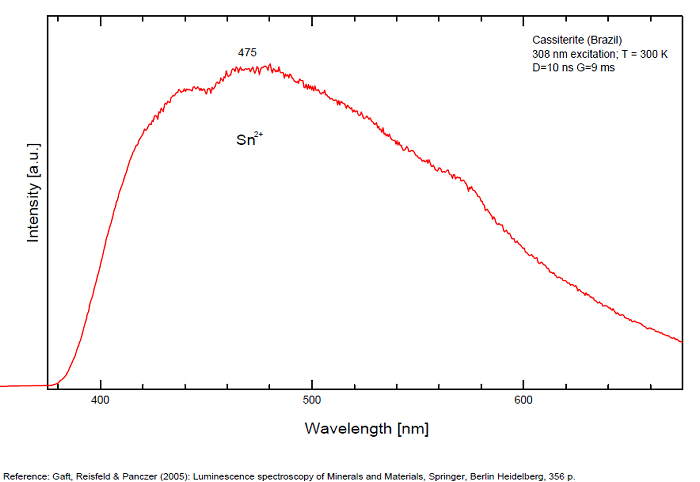
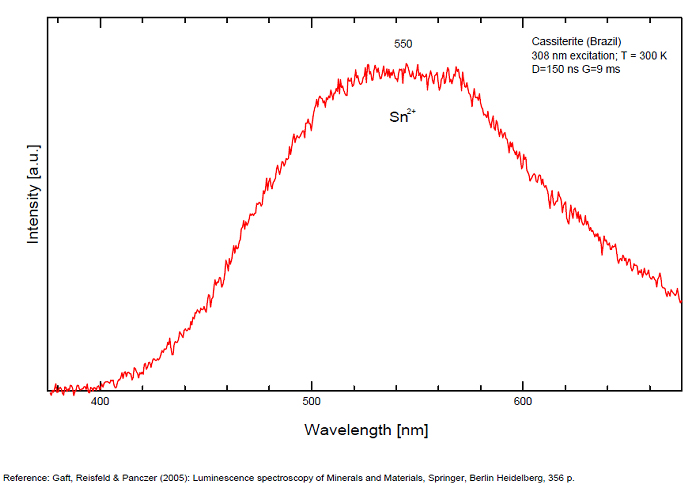
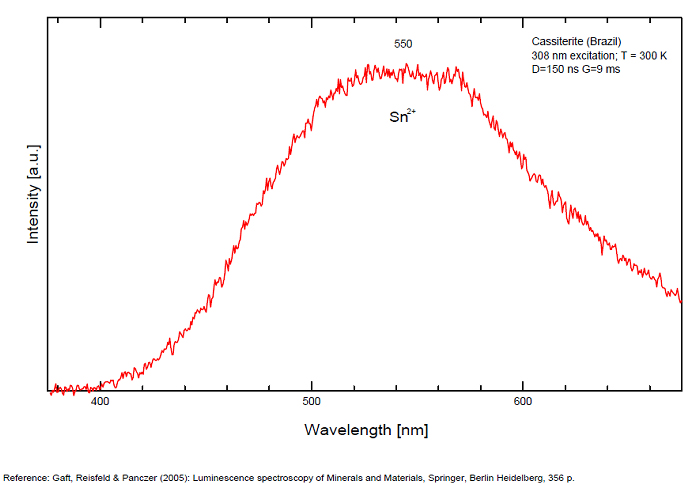
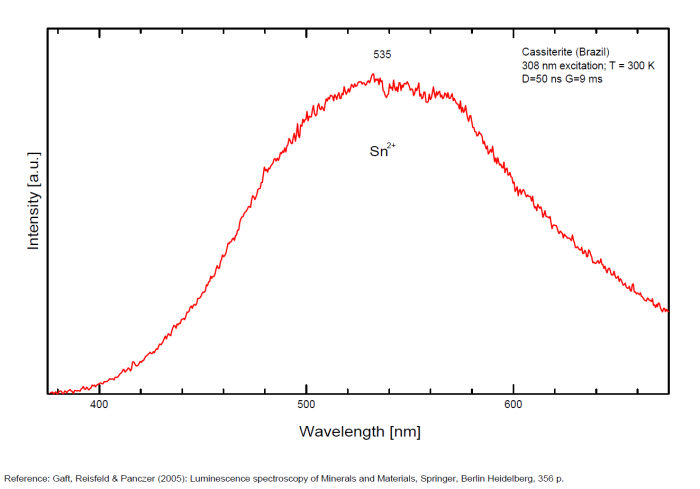
CASSITERITESnO2
Activator(s):
Sn2+,
Other activators:
Sn3+, W,
Peaks in the spectrum (nm):
Sn2+ : very large band at 475nm (200nm half-width) Sn2+ : very large band at 535 - 537nm (140nm half-width) Sn2+ : very large band at 550 nm (140nm half-width)
Comments on spectra and activators:
Blue luminescence: activator W Yellow luminescence: Ti Under UV lamp excitation cassiterite samples are luminescent only at low temperatures. But with powerful laser excitation (337 nm) even at 300 K or at low temperature (77k) bright intrinsic luminescence (Sn2+ and Sn3+) (Gaft) The spectrum under powerful laser at 300K is similar to those at 77 K with lamp excitation. Decay time of luminescence has two components, namely 100 ns and 280 ns (Gaft and Vorontsova 1982; Gaft et al. 1988).
(Based on Cathodoluminescence, Waychunas)
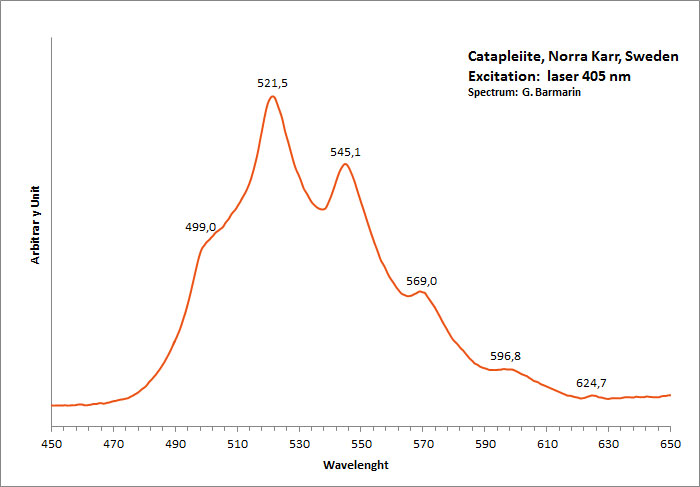
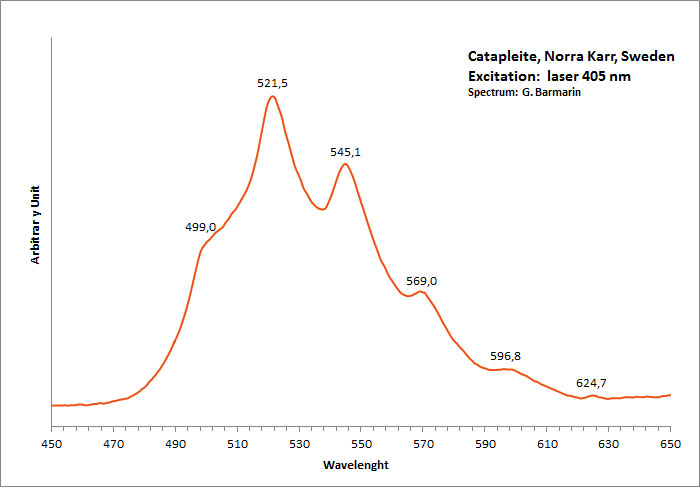
CATAPLEIITENa2ZrSi3O9 2H2O
Activator(s):
n[TiO6] cluster,
Other activators:
(UO2)2+ (ion Uranyle) en impureté,
Peaks in the spectrum (nm):
(UO2)2+ : 499, 521, 544, 570, 597nm Very large band with max at 550 nm (Gorobets)
Comments on spectra and activators:
Blue luminescence in catapleite due to TiO6 (Gaft); Cathodoluminescence: light-blue;
Green luminescence due to Uranyl impurities (Typical spectrum);
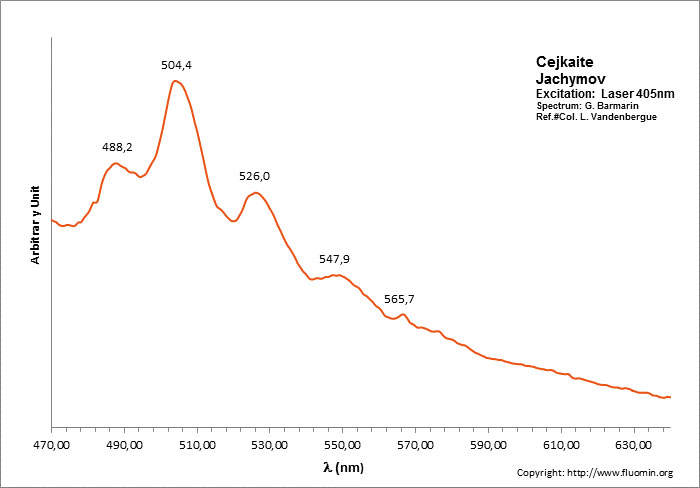
CEJKAITENa4(UO2)(CO3)3
Activator(s):
(UO2)2+ (ion Uranyle) intrinsèque ,
Peaks in the spectrum (nm):
466, 486, 505, 525, 547, 570, 597nm
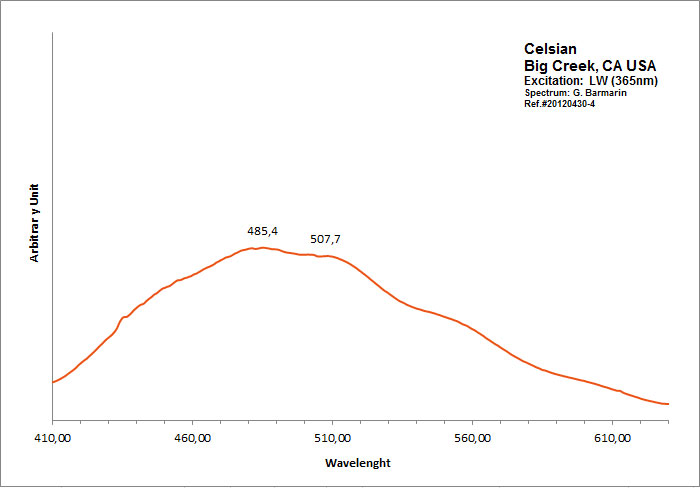
CELSIANBaAl2Si2O8
Activator(s):
,
Peaks in the spectrum (nm):
Large spectrum peaking around 485nm
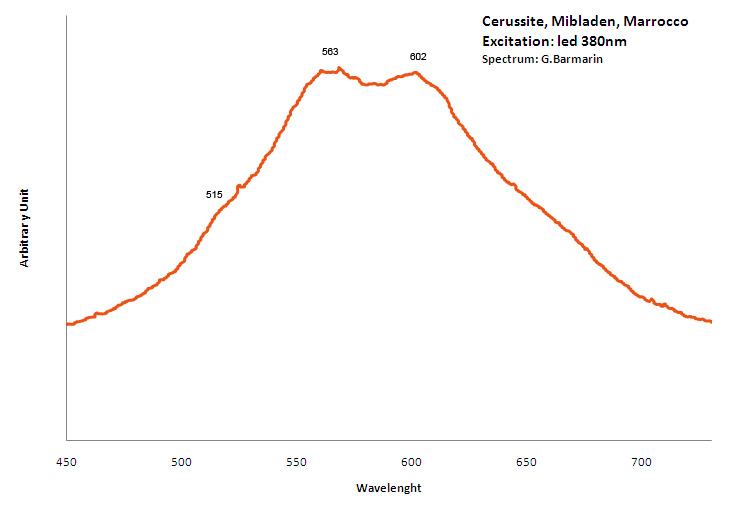
CERUSSITEPbCO3
Activator(s):
Pb2+,
Other activators:
Sm3+, Eu3+, Dy3+, Ag+,
Peaks in the spectrum (nm):
(?) : large band peaking at 515 nm, Sm3+ : peaks at 564, 597, 649nm Dy3+ : 574, 577nm Nd3+ : 876, 919nm Eu3+ : 615, 621, 643, 657, 704nm
Comments on spectra and activators:
Lifetime: 218μs @475nm; Excitation by CW laser with 532 and 780 nm revealed several luminescence bands and lines. IR lines under 780 nm excitation may be evidently ascribed to Nd3+ and other trivalent REE luminescence present under 532 nm excitation, while the bands origin needs further investigation. (Gaft)
chalcedony
Activator(s):
(UO2)2+ (ion Uranyle) en impureté,
Peaks in the spectrum (nm):
(UO2)2+ : 502nm, 521nm, 544nm, 568nm, 601nm (fluo green) 569nm, 601nm, 647nm (fluo yellow)
The specified directory does not exist.
CHERNIKOVITE(H3O)2(UO2)2(PO4)2 6H2O
Activator(s):
(UO2)2+ (ion Uranyle) intrinsèque ,
Peaks in the spectrum (nm):
(UO2)2+ : 484.9, 502.1, 523.6, 547.5, 573, 602.8nm Synthetic Chernikovite : 505, 526, 550, 575,615nm
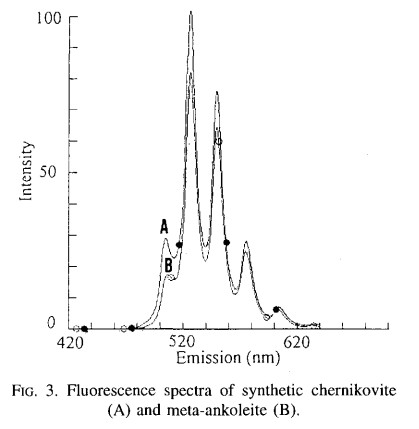
CHKALOVITENa2BeSi2O6
Activator(s):
(UO2)2+ (ion Uranyle) en impureté,
Peaks in the spectrum (nm):
UO22+ : 500, 518, 540nm
Comments on spectra and activators:
Seems to be similar to Uranyl spectrum associated with impurities.
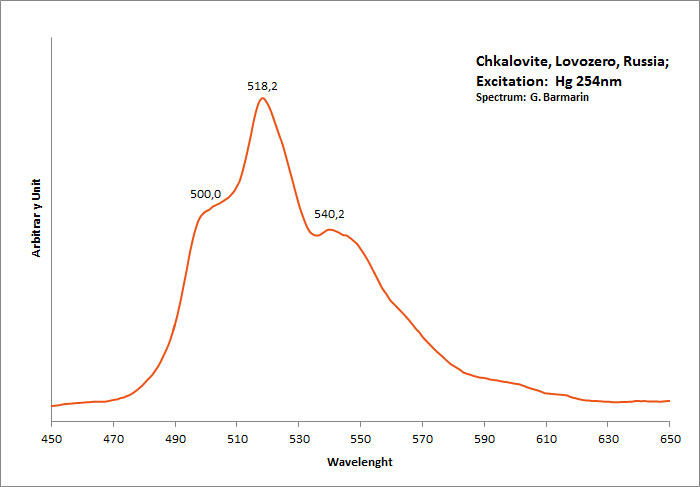
CHONDRODITE(Mg,Fe+2)5(SiO4)2(F,OH)2
Activator(s):
,
Peaks in the spectrum (nm):
Broad band peaking around 570-580nm
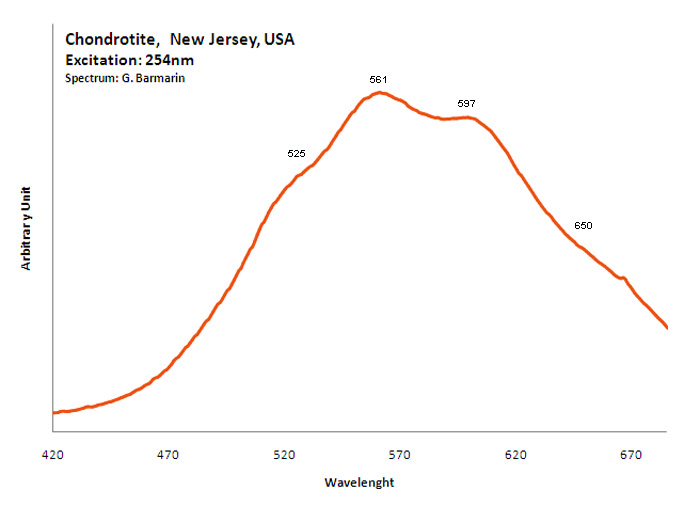
CHRYSOBERYLBeAl2O4
Activator(s):
Cr3+,
Other activators:
V2+,
Peaks in the spectrum (nm):
Cr3+ replacing Al : 678, 680 nm V2+ : band around 720 nm (80nm half-width) (Gaft), VO6 : broad band at 645nm (FWHM: 120nm) TiO6 : 516nm (FWHM: 115nm) (Gaft), band at 540 nm (Gorobets), 689, 698, 703nm (Gaft)
Comments on spectra and activators:
The Al3+ ions are octahedrally coordinated by the oxygen ions and occur in two not equivalent crystal field sites in the lattice. The Cr3+ ions replace substitutionally the Al3+ ions, 78 % replacing Al3+ ions in one position and the rest going into the second sites. The major features of the spectra at 300 K are the two sharp lines R1m (m for mirror, the first position for Al3+ replacement) and R2m peaking at 680 nm and a broad, structured band peaking at lower energies. The 689.5 and 695.2 nm decay time is much larger than that for the 677.9 and 679.5 nm emission (Suchoki et al. 1987). At certain excitation wavelengths, such as 488nm, the R1i (i for inversion) and R2i peaking at 690.0 and 695.0 nm lines associated with Cri ions in inversion sites (second site) appear (Powell et al. 1985) The Cr3+ luminescence properties in natural chrysoberyl minerals have been studied as a function of the Cr content as well as impurities such as Fe and V. A competition was found between Cr and V for very low Cr concentration with the vanishing of Cr3+ emission from Cr3+ ions located in inversion site. The Fe3+ ions substitute in mirror site efficiently with a strong impact on the Cr3+ lifetime of mirror site (Ollier et al. 2015). Cathodoluminescence: red;
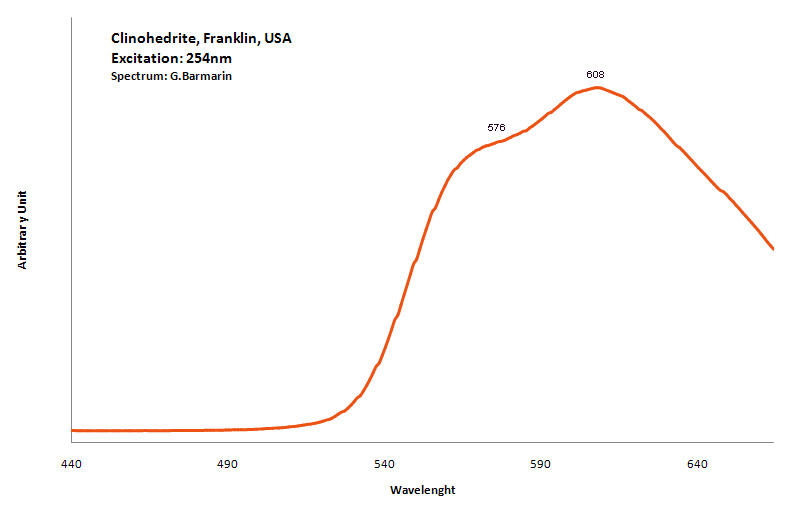
CLINOHEDRITECaZnSiO4 H2O
Activator(s):
Mn2+ ,
Peaks in the spectrum (nm):
Mn2+ : very broad band peaking at 576nm (70nm half-width)
Mn2+ : very broad band peaking at 608nm (200nm half-width)
Comments on spectra and activators:
Activator is Mn2+ substituting to Ca2+;
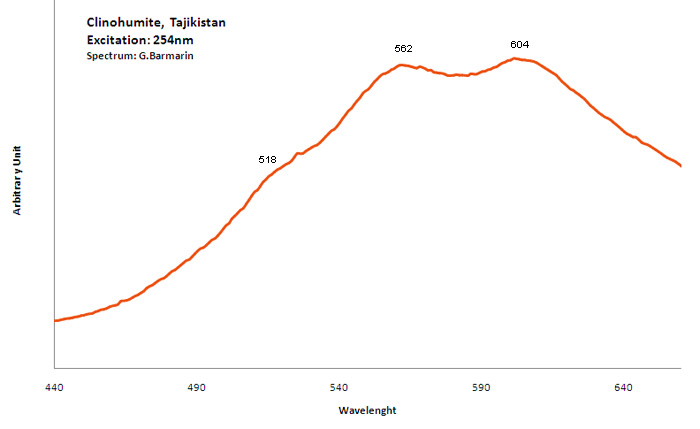
CLINOHUMITE(Mg,Fe+2)9(SiO4)4(F,OH)2
Activator(s):
Mn2+ ,
Other activators:
Fe3+, TiO6, n[TiO6] cluster,
Peaks in the spectrum (nm):
TiOn (Ti4+ - Si4+) : 500nm n[TiOn] : 550-600nm Mn2+ repl. Mg2+ : 630 - 650nm Fe3+ : broad band at 745nm OH (?) : 660nm (peak)
Comments on spectra and activators:
Steady state luminescence of clinohumite was connected to Mn2+ and possible TiO6 centers (Gorobets and Rogojine 2001). Excitation by CW laser with 532 nm revealed two emission bands which may be ascribed to Mn2+ and Fe3+ (Gaft)
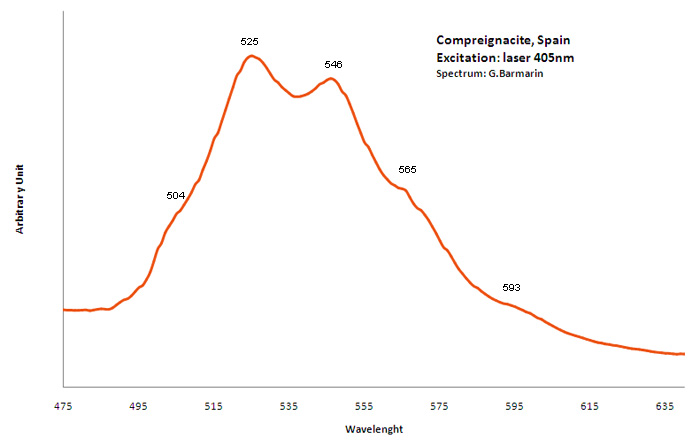
COMPREIGNACITEK2(UO2)6O4(OH)6 7H2O
Activator(s):
(UO2)2+ (ion Uranyle) intrinsèque ,
Peaks in the spectrum (nm):
(504nm), 525nm, 546nm, 585nm, (593nm) / 500.7, 516.1, 532.4, 554.3, and 579.6 nm (Thuro Arnold and Nils Baumann)/
Comments on spectra and activators:
fluorescence lifetimes of 202 and 914 ns (bi-exponential)
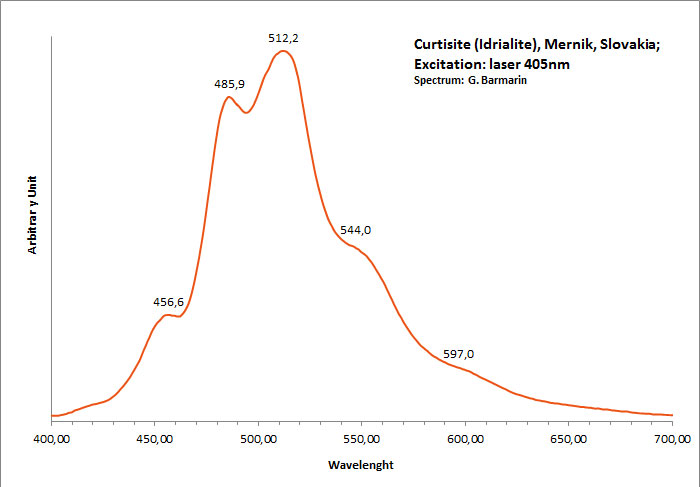
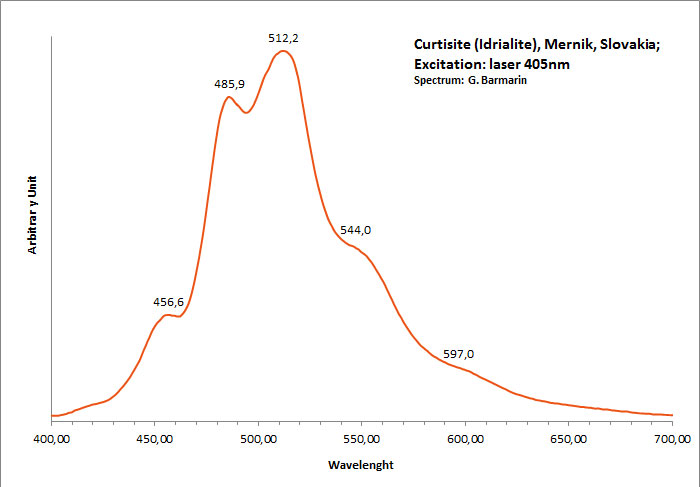
curtisite
Activator(s):
Matière organique intrinsèque,
Peaks in the spectrum (nm):
Peaks at: 457nm, 486nm, 512nm, 544nm, 597nm ;
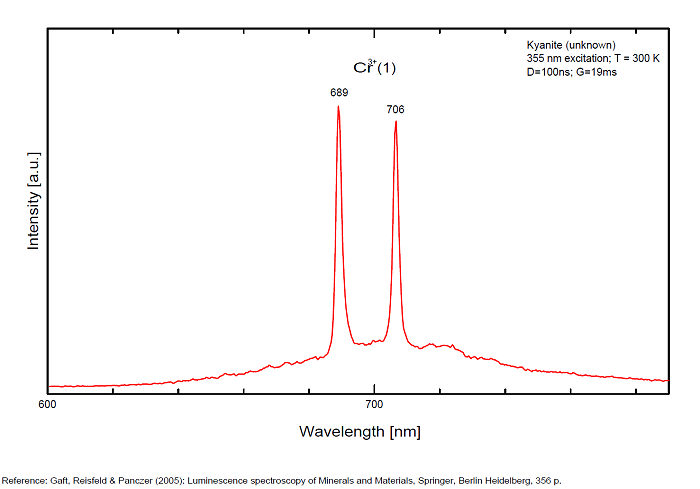
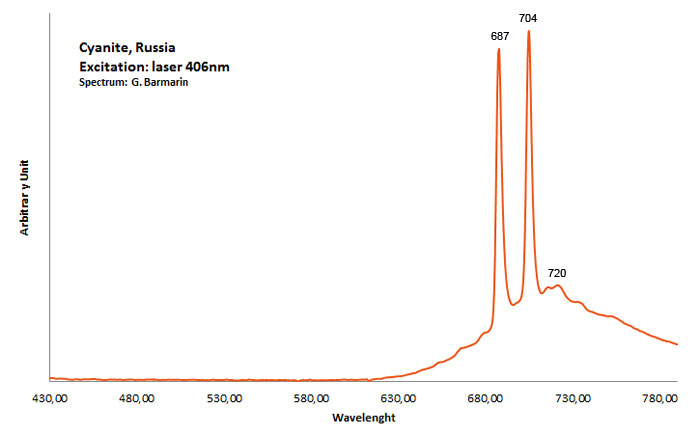
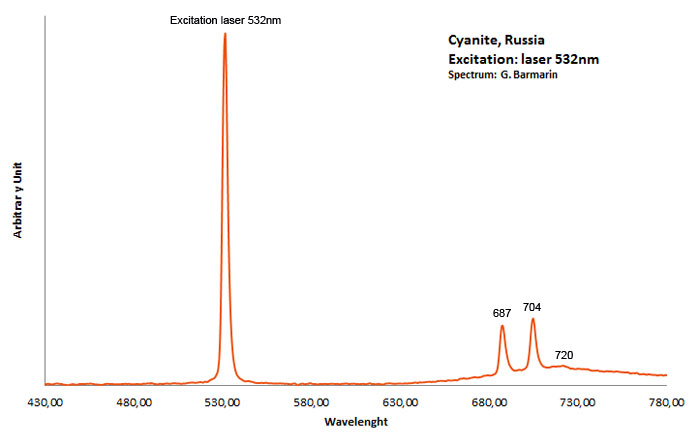
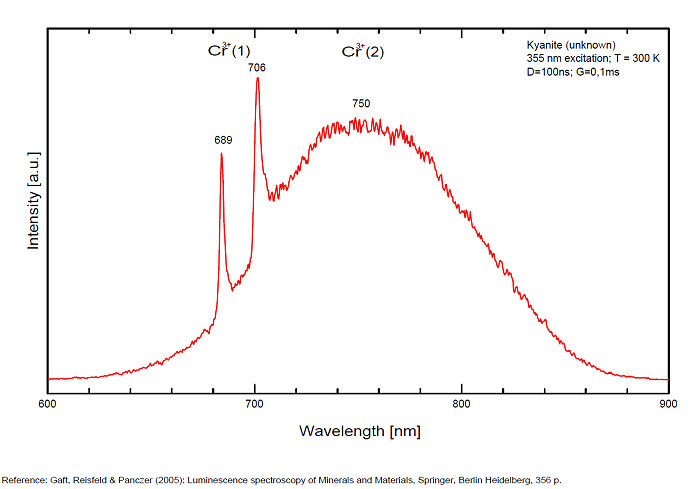
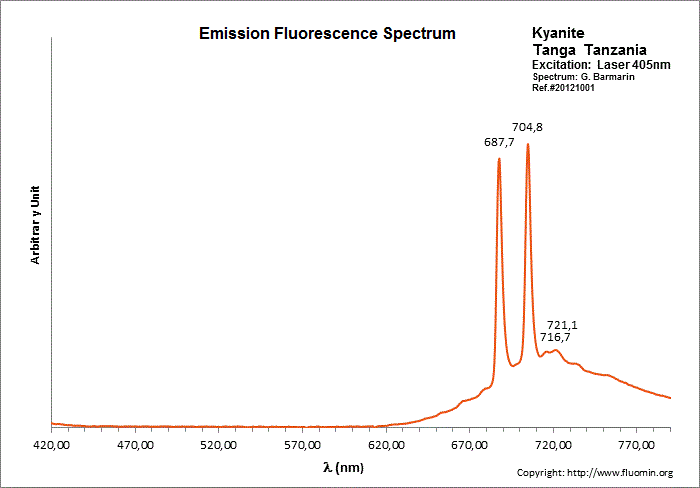
CYANITEAl2SiO5
Activator(s):
Cr3+,
Other activators:
TiO6,
Peaks in the spectrum (nm):
Cr3+ replacing Al (site A giving Doublet R-lines) : Lines at 688 or 689 and at 706nm Cr3+ (site B weak crystal field) : broad band peaking at 750nm Cr3+ (site C weak crystal field) : broad band peaking at 780-790nm (Gaft) TiO6 : very large band at 500-570nm (Gorobets)
Comments on spectra and activators:
Kyanite has four inequivalent Al3+ sites, which may be substituted by Cr3+. Red luminescence reported to Cr3+ replacing aluminium (Tarashchan in Gaft) but three different site for Cr3+ identified. The study of Cr3+ luminescence in kyanite has long history.

DANBURITECaB2(SiO4)2
Activator(s):
Eu2+,
Other activators:
(UO2)2+ (ion Uranyle) en impureté, Ce3+, Sm3+, Eu3+, Dy3+, Tb3+, Mn2+ , Nd3+, Yb2+,
Peaks in the spectrum (nm):
Ce3+: 346 - 347, 367nm
Eu2+: 437 - 438, 455nm
Yb2+: 535nm
Eu3+: 580, 592, 610 - 611 - 613, 618 - 620, 654, 690nm
(All REE replacing Ca2+)
Comments on spectra and activators:
Triboluminescence cited by De Ment (1947). Luminescence centers Eu2+, Yb2+, Ce3+, Dy3+ and Sm3+ characterize steady-state spectra of danburite (Gaft et al. 1979).






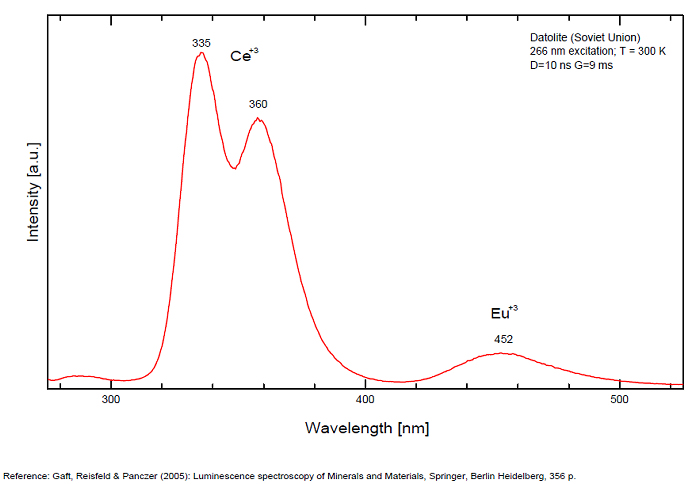
DATOLITECa2B2Si2O8(OH)2
Activator(s):
Eu2+,
Other activators:
Ce3+, Eu3+, Mn2+ , Yb2+,
Peaks in the spectrum (nm):
Ce3+: 335, 350, 360, 370nm
Eu2+: 430, 445, 455nm
Yb2+: 520nm
Mn2+: 565nm
Eu3+: 577, 598, 510, 616 - 617, 700nm
(All repl. Ca2+)
Comments on spectra and activators:
Luminescence centers Eu2+, Yb2+ and Mn2+, characterize steady-state spectra of datolite (Gaft et al. 1979). By using laser-induced time-resolved spectroscopy Gaft and al. were able to detect the following emission centers: Mn2+, Ce3+, Eu2+, Eu3+, Sm3+, Dy3+
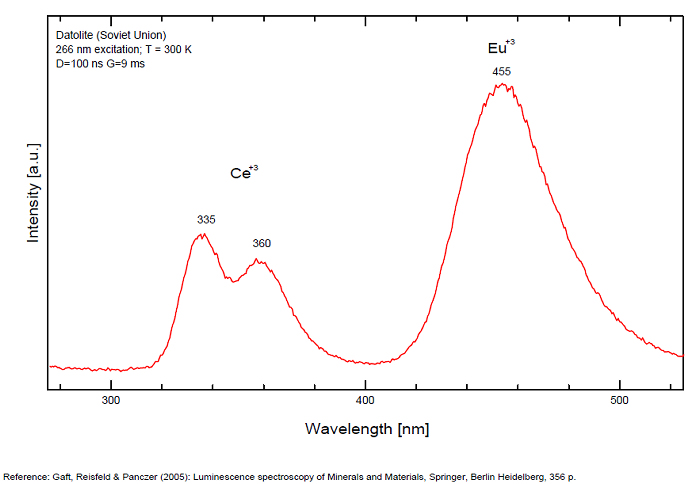
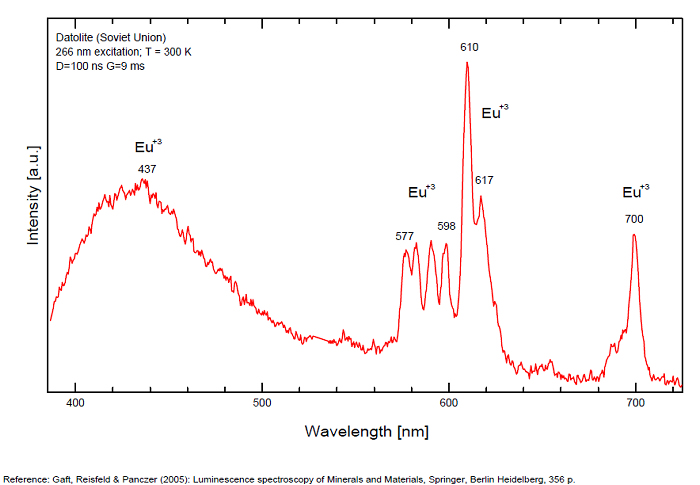
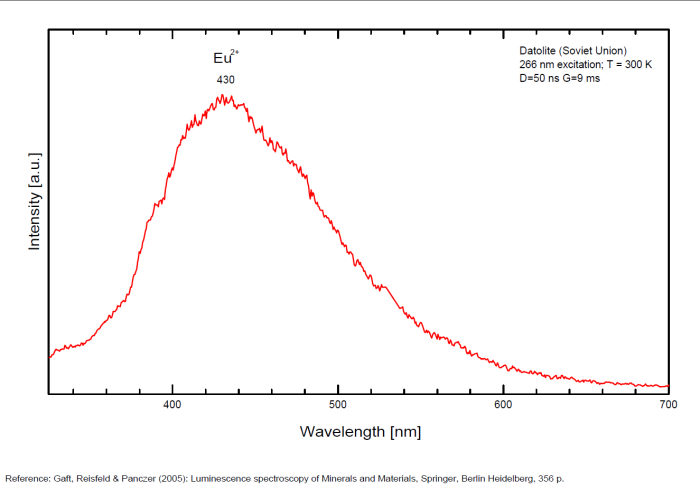
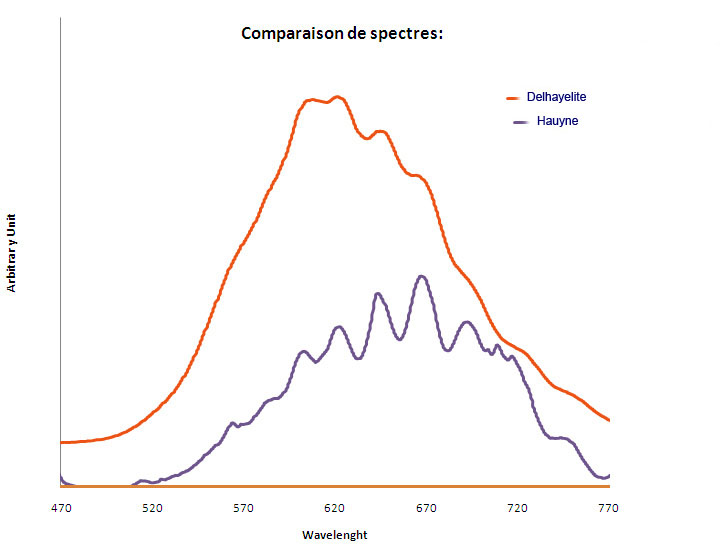
DELHAYELITE(Na,K)10Ca5Al6Si32O80(Cl2,F2,SO4)3 18H2O
Activator(s):
S2-,
Other activators:
Ce3+, Sm3+, Eu3+, Dy3+, Mn2+ , Nd3+,
Peaks in the spectrum (nm):
S2- : large band centered at 620nm with weak vibrational structure (564, 584, 608, 622, 645, 664, 692, 719, 747nm)
Comments on spectra and activators:
Delhayelite was studied by steady-state luminescence and S2- , trivalent REE, such as Ce, Dy, Sm, and Mn2+ were found. Excitation by CW laser at 532 and 785 nm revealed trivalent REE, mainly Eu and Nd, and evidently S2- band (Gaft).
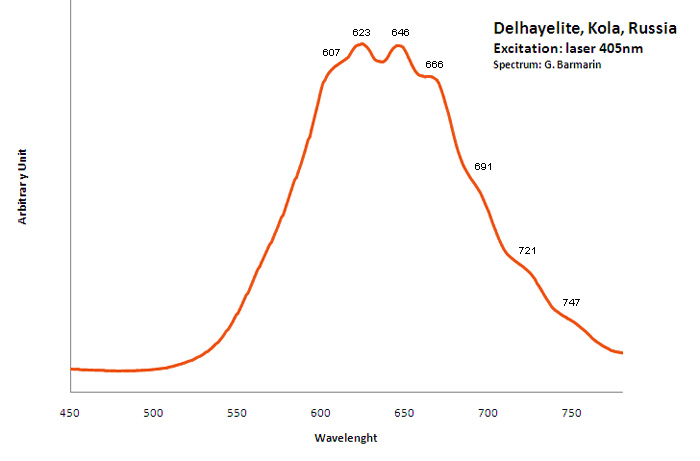
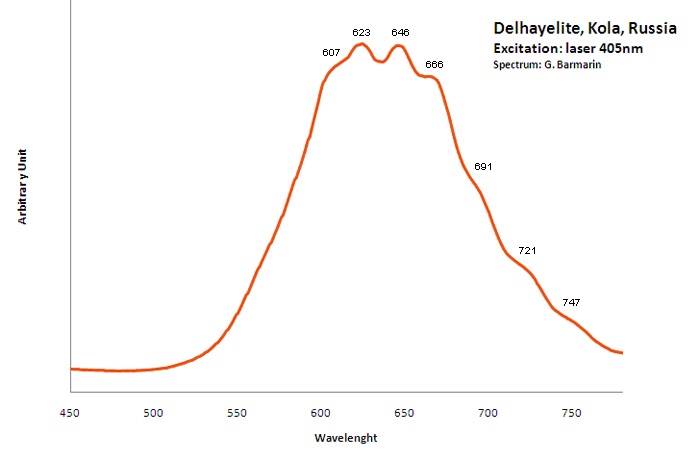
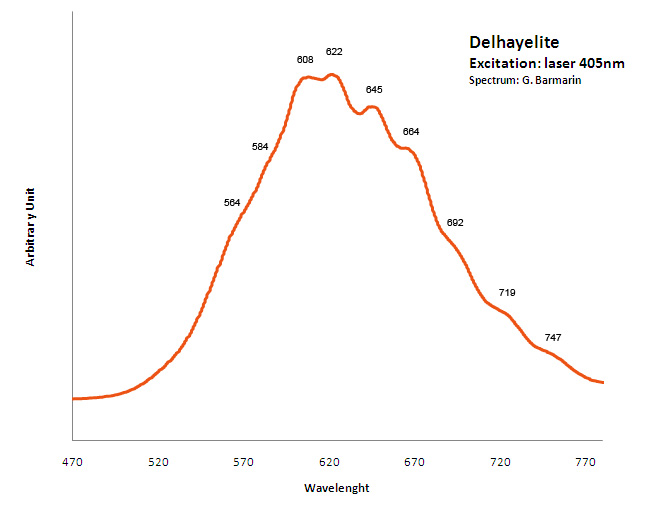
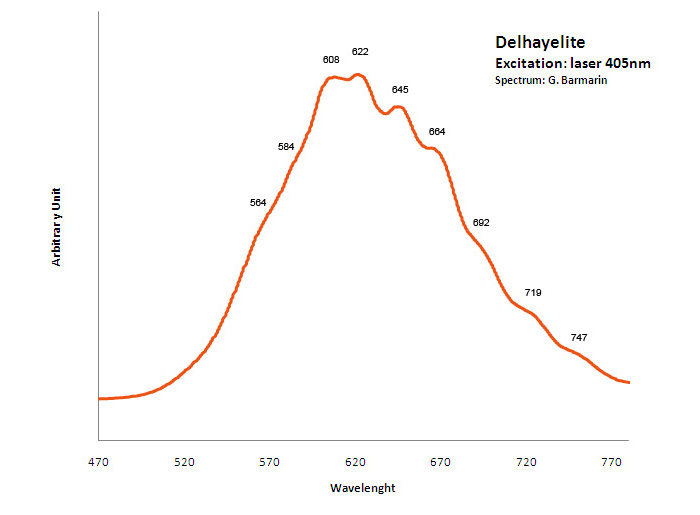
Excitation: laser 405nm. Col. G. Barmarin; Spectre: G. Barmarin
DIAMONDC
Activator(s):
N (Azote),
Other activators:
B (Bore),
Peaks in the spectrum (nm):
N3 center: 442, 507 nm
H3 center: 520nm
A-band center: 452nm
N3 center: 415, 428, 439, 452, 731nm
Comments on spectra and activators:
From more than 100 detected centers only for seven the models are determined, mainly based on EPR interpretations. The model includes identification of the impurity, vacancy, interstitial atom, their aggregations and their crystallochemical position together with quantum-chemical and spectroscopic description. (Gaft) Luminescence of diamond is related to various defects in its structure, almost always related to N (Nitrogen) atoms. It is logical, because the atomic radii of C and N are nearly equal (approximately 0.77 Å). N3 center: color and luminescence center attributed to 3 Nitrogen atoms bounded to the same Carbon atom or a vacancy. The luminescence spectra of this center is associated with a large peak at 440-450nm with a fine structure (peaks around 415, 428, 438 and 451nm) a lifetime: 30-40ns and an absoption energy of 2,985eV but other centers (Al imputity for exemple) contribute to this spectrum even if it is not possible aboce 170K to discriminate their contribution. H3 and H4 centers: 496, 506, 512nm ; two Nitrogen atoms coupled with a vacancy in two different configurations. Absorption energy: 2,463eV. Decay-time: 15-20ns S1 center: a combination of one vacancy with one Nitrogen atom, 541nm S2 center: a combination of two vacancies with one Nitrogen atom, 565nm S3 center: a combination one vacancy with several Nitrogen atom H3, H4, S2 and S3 centers are characterized by broad bands at 520-545nm with very weak lines at 489nm and 523nm (S2), 498nm (S3) and 503nm (H3) A-band at 480nm observed in natural diamond of type I A-band with a maximum at 440 nm in natural diamond of type II (low Nitrogen diamonds) Some blue diamonds exhibit a remarkable red phosphorescence such as Hope or Wittelsbach-Graff (both of Indian origin) and Blue Moon (South Africa Cullinan Mine); All natural, untreated type IIb blue diamonds (diamond without Nitrogen but containing some uncompensated Boron) show a phosphorescence with two emission bands: one at 660 nm and another at 500 nm but for those three diamonds, unlike usually, it is the blue that decreases more quickly than the red causing this magnificent red phosphorescence. Type IIb diamonds with orange-red phosphorescence are more commonly originated in India or Venezuela but not in the case of the blue Moon coming from the Cullinan Mine in South Africa where diamond are normally found with a more typical brief bluish phosphorescence. After exposure to short-wave ultraviolet light, the Blue Moon display orange-red phosphorescence (around 660nm) that remain visible for up to 20 seconds. (see bibliography: Eloise Gaillou, Gems and Gemmology, Winter 2014)
The specified directory does not exist.
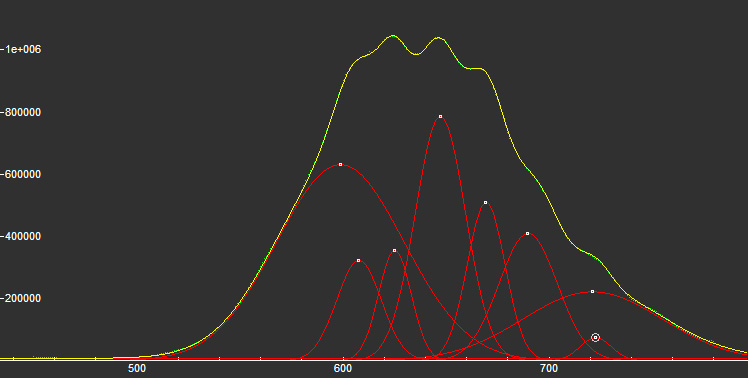
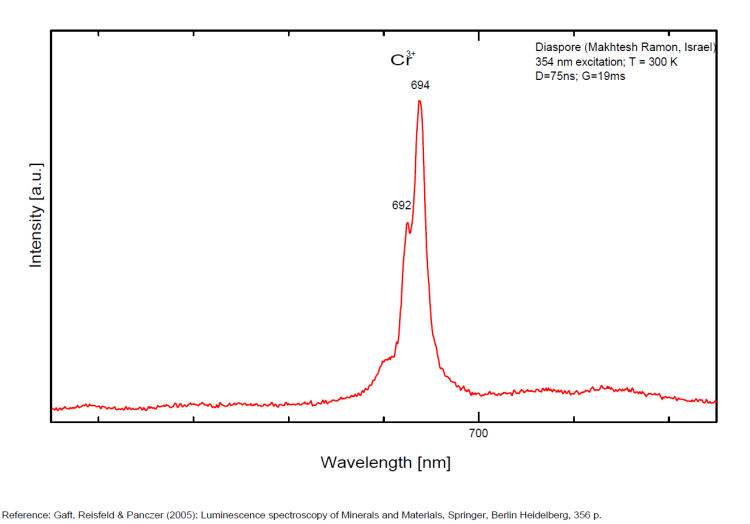
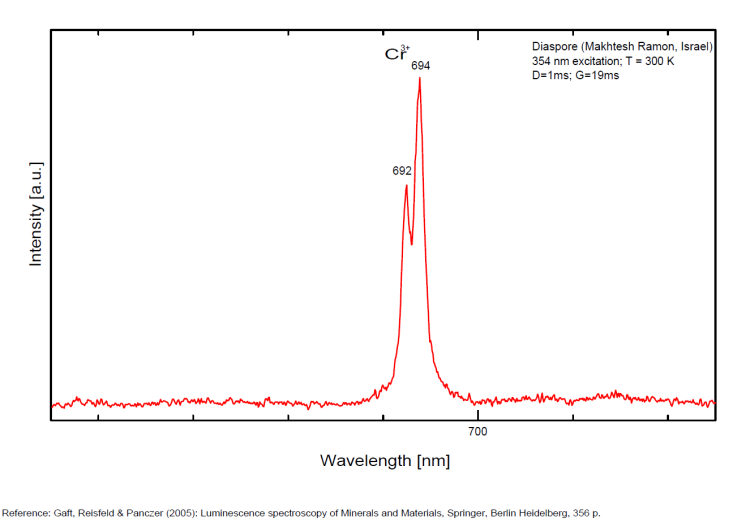
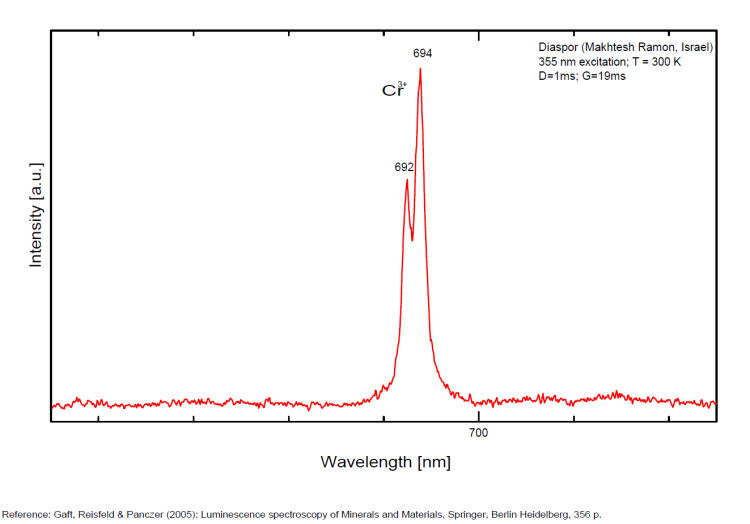
DIASPOREAlO(OH)
Activator(s):
Cr3+,
Peaks in the spectrum (nm):
Comments on spectra and activators:
Laser induced luminescence shows lines for Cr3+ at 692 and 694 nm, typical of 2E→4A2 transitions of Cr3+ luminescence centers (R-lines) substituting for Al3+ in six-fold coordination.
Yellow luminescence activator not known
DUMORTIERITE(Al,Fe3+)7(SiO4)3(BO3)O3
Activator(s):
TiO6,
Other activators:
Cr3+,
Peaks in the spectrum (nm):
TiO6 replacing AlVI3+ : Large band peaking at 460 nm
Cr3+ replacing AlVI3+ "R-Lines": 680, 688 nm (Gorobets)
455, 487, 508, 525 and 545nm
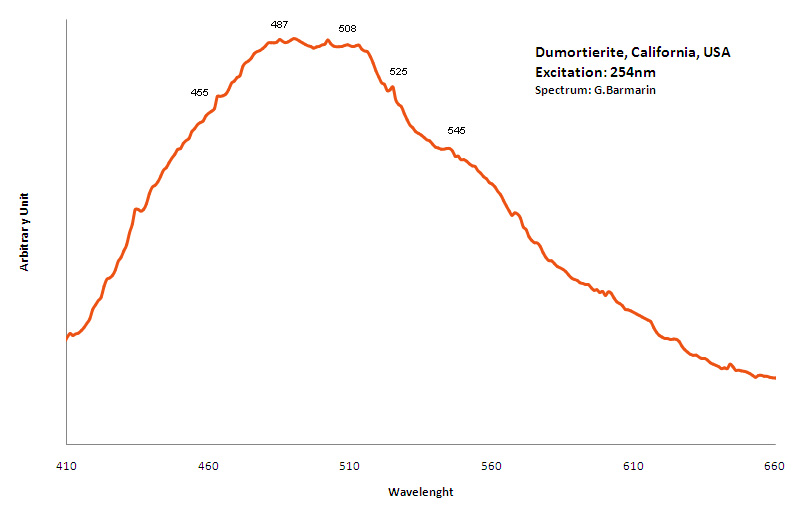
emerald
Activator(s):
Cr3+,
Other activators:
Fe3+, Mn4+, Mn2+ , V2+,
Peaks in the spectrum (nm):
Cr3+ : Lines at 682, 683, 684nm, Cr3+ replacing Al3+ : broad band peaking at 730nm Cr3+ : (666), 685, (696), Cr3+ I: 725nm (Band) Cr3+ II: 715nm (Band) Fe3+ : broad band at 735nm Cr3+ , V2+, Mn4+ : broad band at 720 (Lifetime = 100 microsecondes) VO4 : 423 (Lifetime = 1 microseconde)
Comments on spectra and activators:
Steady-state emission of beryl was previously studied. The broad band at 720 nm is connected with Fe3+, while the relatively narrow bands at 480 and 570 nm are ascribed to Mn2+ in tetrahedral and octahedral coordination, correspondingly. Cr3+ emission was connected with narrow R-lines at 680 and 682 nm (Tarashchan 1978; Kuznetsov and Tarashchan 1988). (Cr3+ impurity ions in highly distorted octahedron sites) The Cr3+ luminescence properties in natural beryl minerals have been studied as a function of the Cr content as well as impurities such as Fe and V. It appears that the Cr3+ crystal field is linked to the Cr amount and decreases when Cr increases. A competition between Cr and V was noticed for very low Cr concentration (Ollier et al. 2015). (Gaft)
The specified directory does not exist.
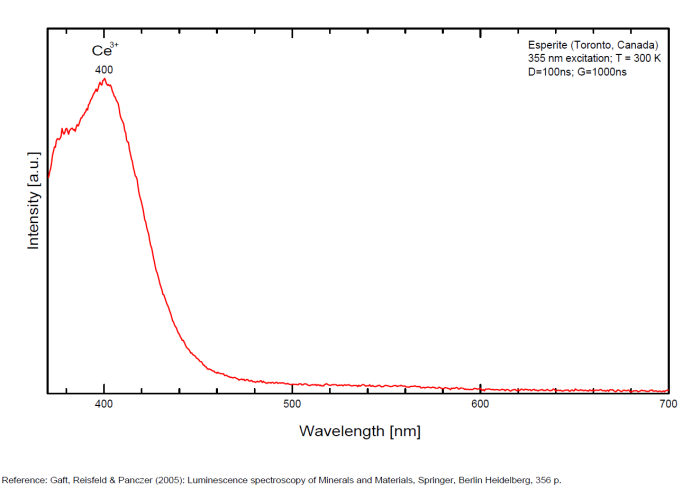
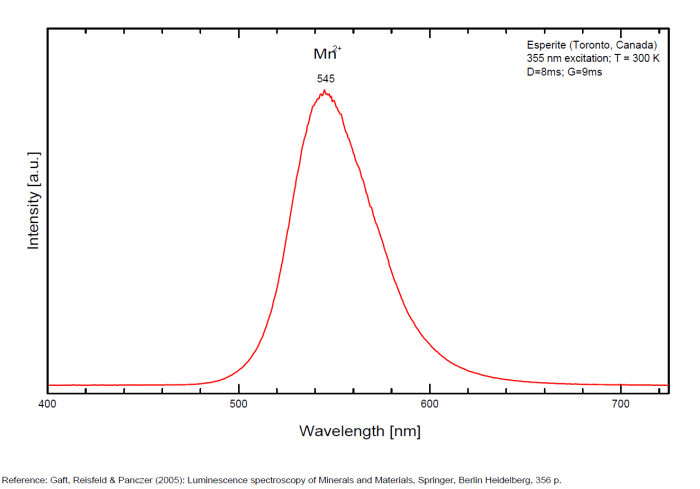
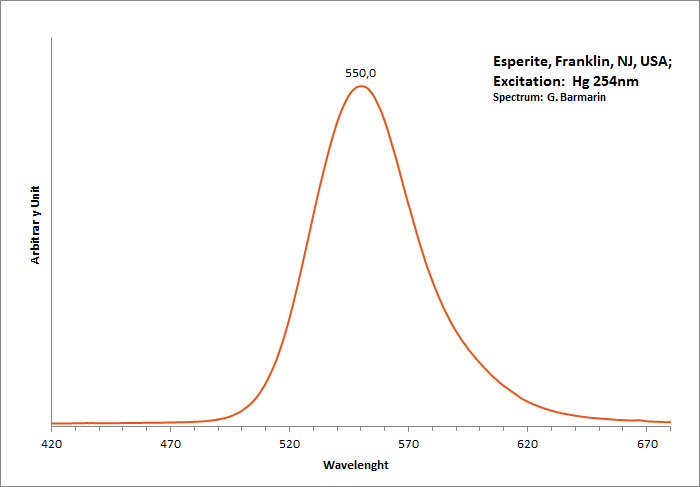
ESPERITEPbCa3Zn4(SiO4)4
Activator(s):
Mn2+ ,
Other activators:
Ce3+, Sm3+, Dy3+,
Peaks in the spectrum (nm):
Ce3+: 400nm
Mn2+ replacing Zn: narrow band peaking at 540-545nm
Sm3+ replacing Ca2+: small peaks at 600, 645nm
Dy3+ replacing Ca2+: 482, 575nm
Reabsorption at 580nm by Nd3+ and at 660nm by UO2+
Comments on spectra and activators:
Activator: Mn2+ (6 coordinated) in Zn and Ca position accompanied by Ce3+,Dy3+ and Sm3+ lines (Gaft).
Decay time: 9 ms
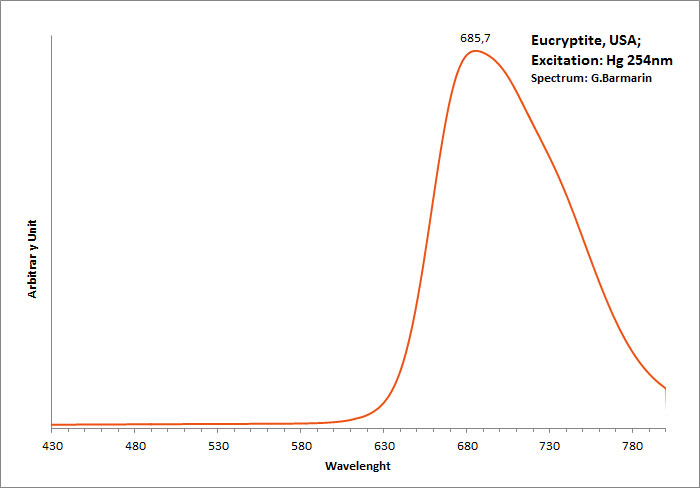
EUCRYPTITELiAlSiO4
Activator(s):
,
Peaks in the spectrum (nm):
685,7nm
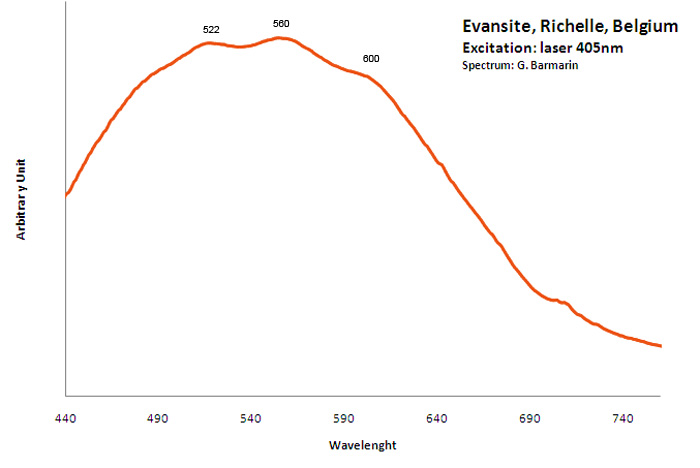
EVANSITEAl3(PO4)(OH)6 6H2O (?)
Activator(s):
(UO2)2+ (ion Uranyle) en impureté,
Other activators:
ST (Singlet-triplet)-Matière organique en impureté,
Peaks in the spectrum (nm):
Organic impurities: Broad band centered around 500-550nm (not related to evansite but calcite from Richelle)
Comments on spectra and activators:
The spectrum shape of classic samples from Richelle combined with strong phosphorescence lets think that the fluorescence is due to organic impurities (but finally those samples seem to be only common calcite!); Green fluorescence is clearly due to uranium in impurities (see bibliography).
feldspar/Group of minerals: ALBITE, MICROCLINE, SANIDINE
Activator(s):
Fe3+,
Other activators:
Pb2+, Eu2+, Ce3+, Sm3+, Dy3+, Tb3+, Mn2+ , Nd3+, Tm3+, Tl+,
Peaks in the spectrum (nm):
Eu3+ : 614, 624, 710nm
Fe3+ : large band at 735 - 741 to 765nm (FWHM: 120nm)
Tm3+ : 798nm
Nd3+ : 895nm
Pb+ : band at 851nm (FWHM : 50nm )
Comments on spectra and activators:
Steady-state luminescence of feldspars is well studied. The following impurity centers have been found: Tl+ , Pb+, Pb2+, REE3+ (Ce, Dy, Sm, Tb, Nd), Eu2+, Mn2+, Fe3+, Cr3+ (Gaft) Luminescence of monovalent Pb was firstly proposed for explanation of IR luminescence band peaking at 860 nm in emission spectrum of feldspars The UV emission of feldspar at 290 nm is linked with the presence of Na and cracks-strain exsolution-twinning; at 340 nm with stress in Si—O bonds; the broad blue band (380-450nm) with [AlO4]o defects; at 560nm with Mn2+ point defects and at 720nm with iron in the lattice. Iron-rich feldspars: luminescence emissions at 560 nm and 750 nm come from point defects of Mn2+ and Fe3+ in feldspar lattices. Furthermore, high amounts of these impurities (e.g. 0.3%) lower these light emissions. Dark and iron-rich samples emit weak luminescence signals.
The specified directory does not exist.
FLUORAPATITECa5(PO4)3F
Activator(s):
Mn2+ ,
Other activators:
(UO2)2+ (ion Uranyle) en impureté, Sm2+, Eu2+, Ce3+, Sm3+, Eu3+, Dy3+, Er3+, Tb3+, Pr3+, Nd3+, Gd3+, Yb3+, Yb2+, Tm3+,
Peaks in the spectrum (nm):
Gd3+: 307,312nm U: 467, 486, 505, 526, 550nm Mn3+: 583nm
Tm3+: 364nm
Ce3+: 375-400
Tb3+: 383, 387, 389, 411, 415, 420, 436, 439, 443, 446nm (in Ca(I) position)
Tm3+: 452,703
Eu2+: 450-460 (in Ca(I) & Ca(II) position)
Mn2+ (repl. Ca2+): 569nm (decaytime: 1,5ms) (in Ca(I) & Ca(II) position & adsorption on carbonate-fluor-apatite surface)
Dy3+: narrow lines at 470, 475, 478, 481, 488,485, 570, 575, 580, 586, 579, 651, 656, 661, 664 nm (in Ca(I) position)
Sm3+: 560, 563, 565, 593, 595,599, 603, 604, 637, 639, 641, 646, 651, 695, 704, 710, 652 nm (in Ca(I) & Ca(II) position)
Ce3+: 543nm (in Ca(I) & Ca(II) position) (decaytime: 20ns)
O2 in channels: 687, 762nm
H2O in channels: 719, 729, 817, 823, 829nm
MnO3-4 repl. PO3-4:, 1143, 1158nm
Eu3+: 573, 616, 631, 578, 608, 615, 627, 579, 590, 618, 653, 700nm (in Ca(I) position)
Sm2+: 689, 691, 695, 704, 708, 711, 716, 724, 726, 733, 736, 749,773, 774, 778, 798, 815nm
Nd3+: 862, 873, 880, 890, 893, 897,910, 1058, 1065, 1070nm (in Ca(I) position)
Yb3+: 993nm (Ca(II) position)
Pr3+: (in Ca(I) position)
Er3+: (in Ca(I) position)
Comments on spectra and activators:
The structure of the apatite is hexagonal, with the symmetry group P63/m. There are two different sites for the Ca2+ in this structure: 40 % of Ca2+ ions are associated with the Ca(I) sites and 60 % are associated with Ca(II) sites. The point group symmetry of the Ca(I) site is C3, with each Ca having six oxygen nearest neighbors that form a distorted triangular prism about the Ca2+ ion. The Ca(II) site has Cs symmetry with the Ca2+ ions sitting at the corners of equilateral triangles with an F ion in the center (see Gaft). In nature, REE can easily substitute for Ca, becoming luminescence centers. With steady-state luminescence spectroscopy, it was discovered that Eu2+, Ce3+, Mn2+, Dy3+, Nd3+, Sm3+ and Sm2+ mainly determine apatite luminescence. It was established that REE3+ have been detected only in the high symmetry Ca(I) position. (see Gaft)














































FLUORAPOPHYLLITEKCa4Si8O20(F,OH) 8H2O
Activator(s):
(UO2)2+ (ion Uranyle) en impureté,
Other activators:
Ce3+, Mn2+ ,
Peaks in the spectrum (nm):
(UO2)2+ : band around 525nm with peaks at 504, 522, 544nm Mn2+ replacing Ca2+ : band peaking at 600-625nm Ce3+ replacing Ca2+ : 343, 355, 365, 372nm [VO4]n- or [TiO4]n- replacing [SiO4] : 500nm
Comments on spectra and activators:
A specimen from the Nadym River area fluoresces red due to manganese activator. From Mont Saint-Hilaire, crystals have been found that fluoresce intense blue like that of Fluorite under SW, MW, and LW (Jacques Poulin). Also Green luminescence due to U impurities in this locality (pics around 525 nm). Two different Mn2+ luminescence centers have been found in steady-state spectra of apophyllite: in Ca position with orange luminescence peaking at 600 - 620 nm and in K position with green emission peaking at 500 nm (Tarashchan 1978 cited by Gaft).
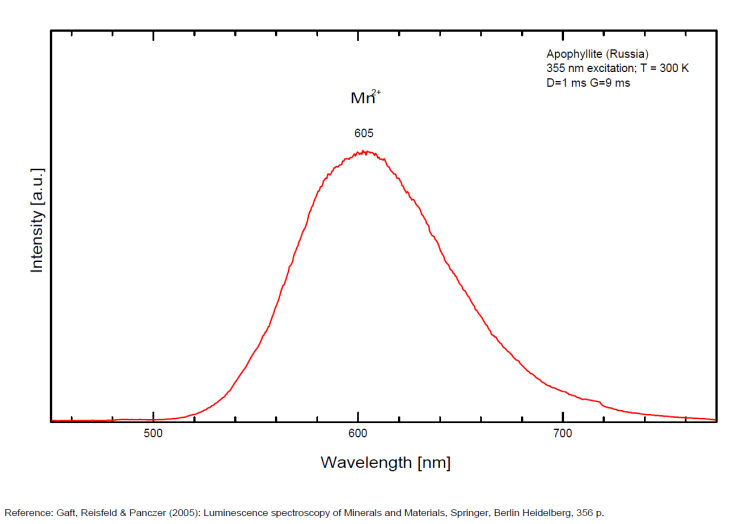
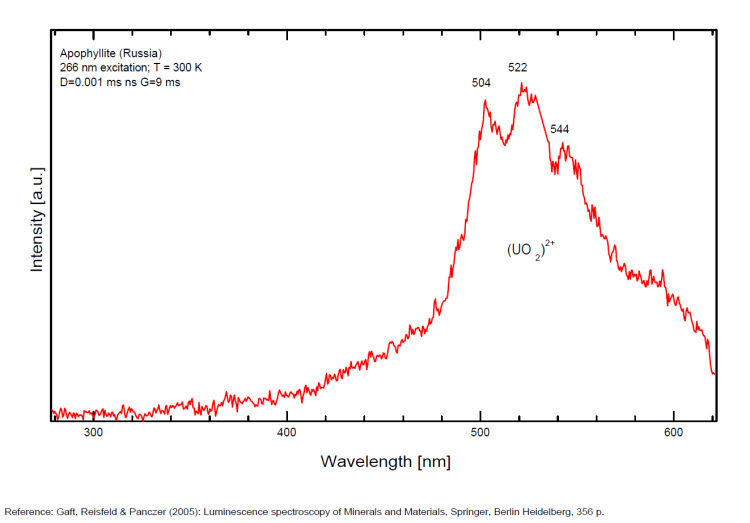
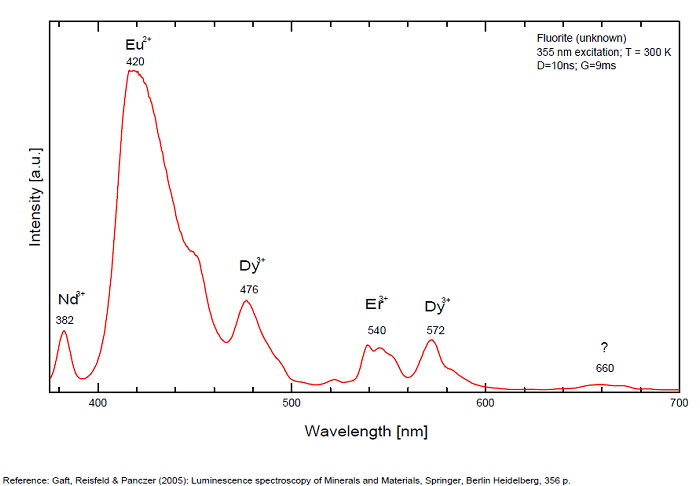
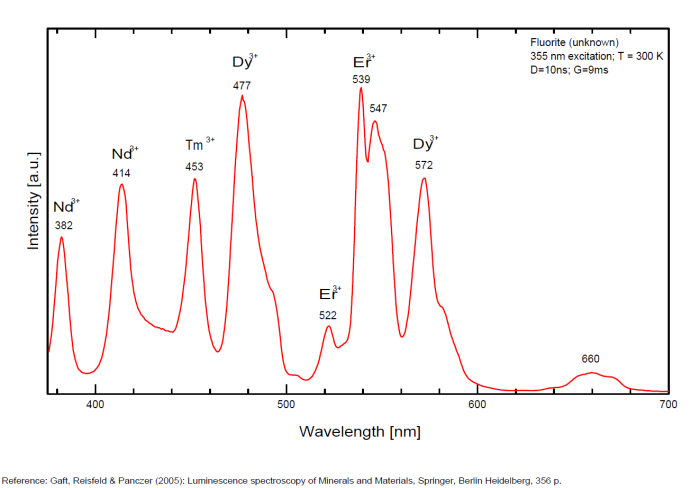
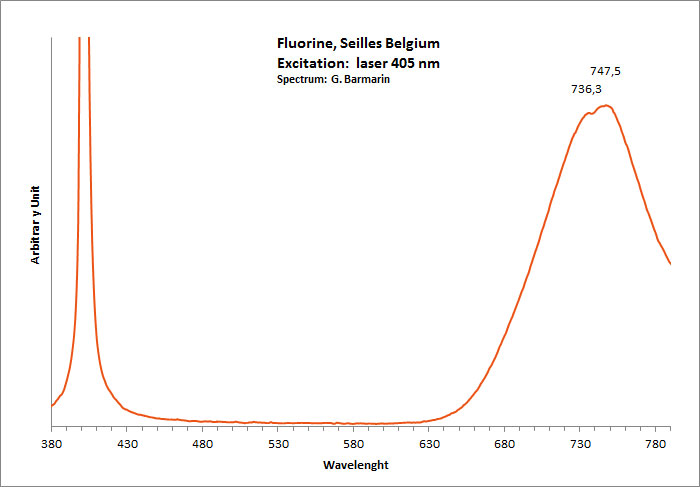
FLUORITECaF2
Activator(s):
Eu2+,
Other activators:
ST (Singlet-triplet)-Matière organique en impureté, Sm2+, Ce3+, Sm3+, Eu3+, Dy3+, Ho3+, Er3+, Tb3+, Pr3+, Nd3+, Yb3+, Tm3+,
Peaks in the spectrum (nm):
Eu2+ repl. Ca2+: 423-425nm (associated with deep blue-violet fluorescence)
Sm2+ repl. Ca2+: 708, 733nm
Yb2+ repl. Ca2+: 550nm
Dy3+:468, 478, 575, 654, 658, 663, 671, 685nm
Tb3+:482, 484, 486, 492, 539, 543, 544, 546, 583, 588nm
Er3+:519, 522, 528, 551, 554nm
Sm3+:561nm
Eu3+: 574, 595, 618, 622, 700nm
Nd3+: 920nm
M-center(2F+Na+): large pic at 720-745 nm
MA center charge stabilized by O2−–F− : 690 nm
MA center charge stabilized by Na+–Ca2+ : 750nm
Comments on spectra and activators:
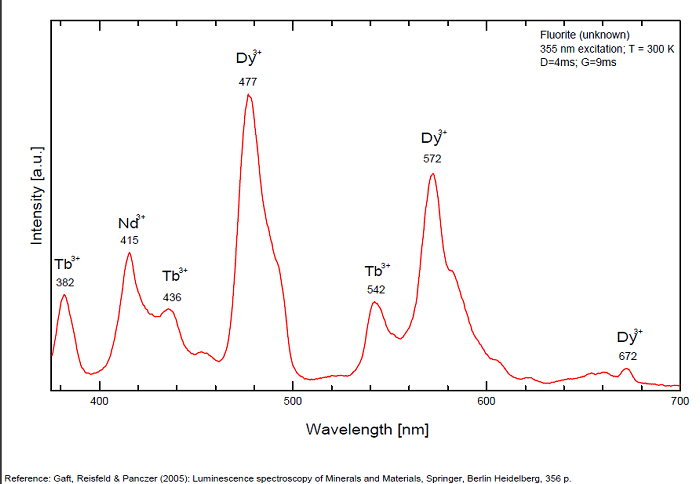
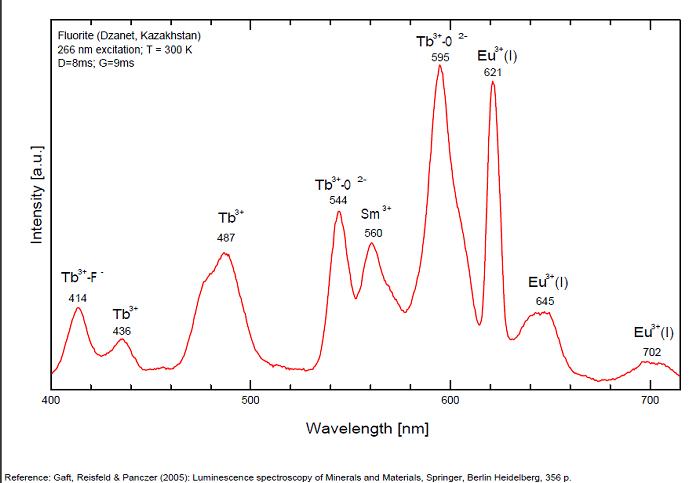
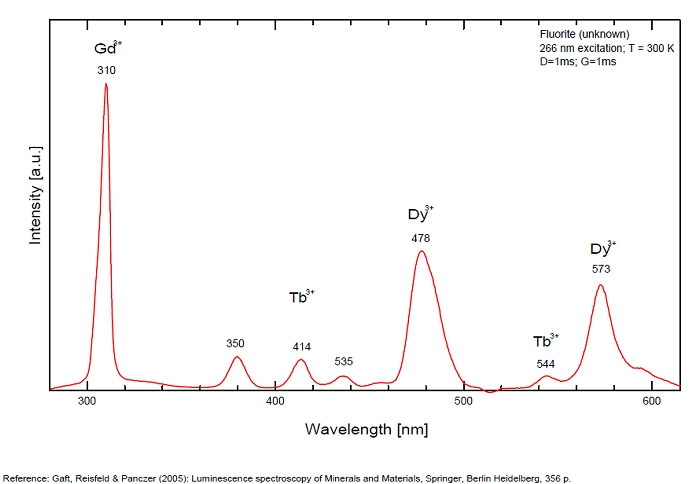
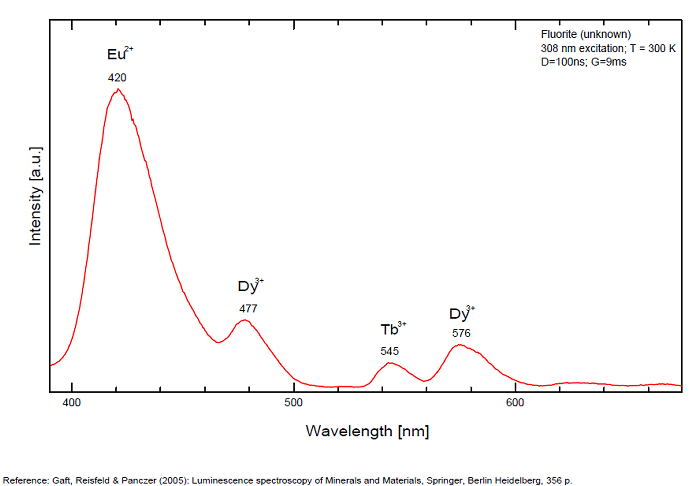
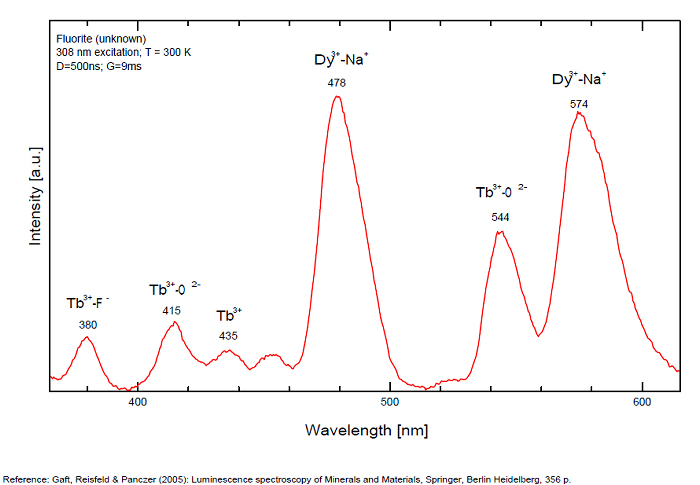
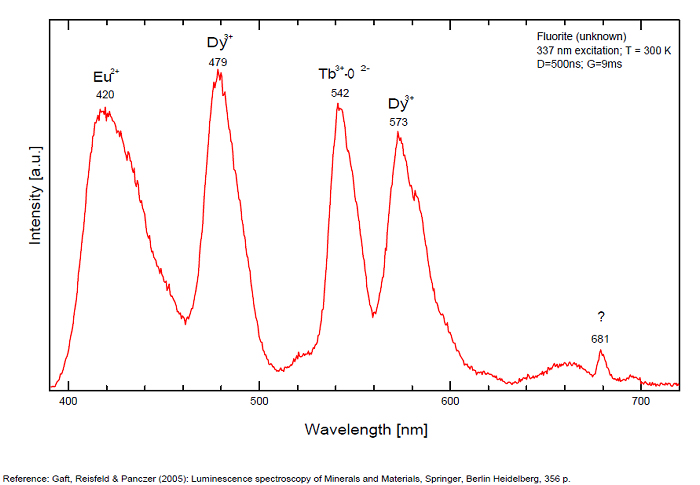
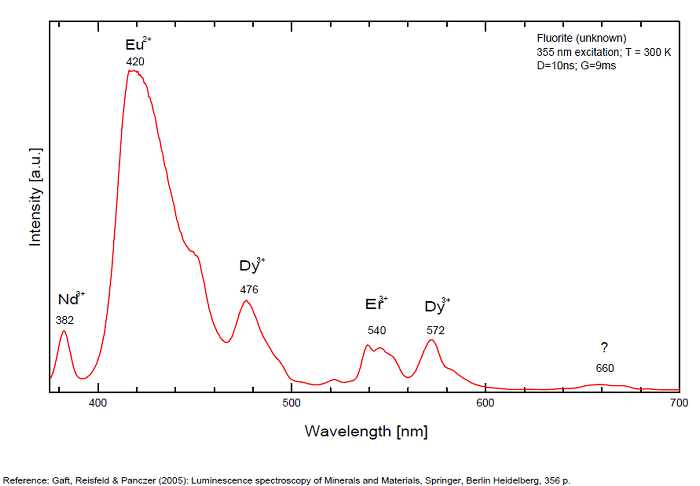
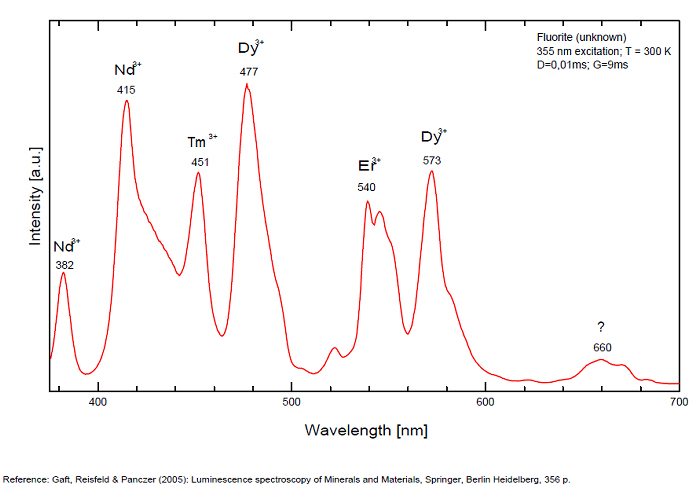
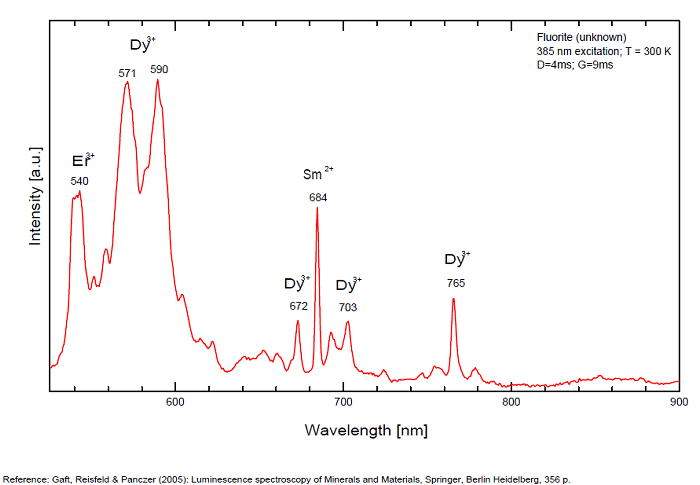
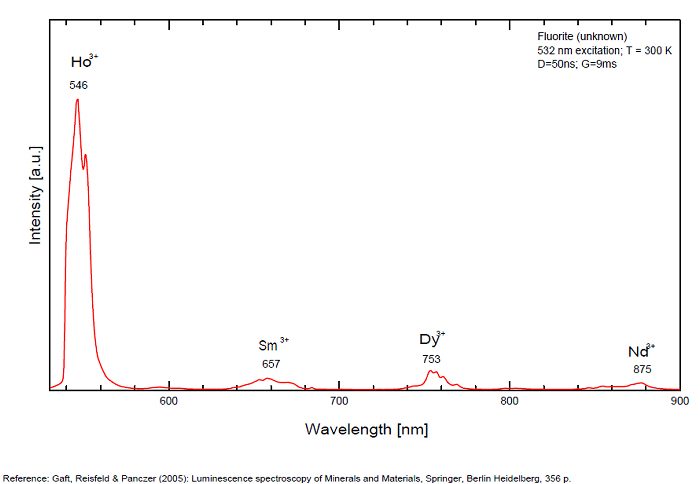
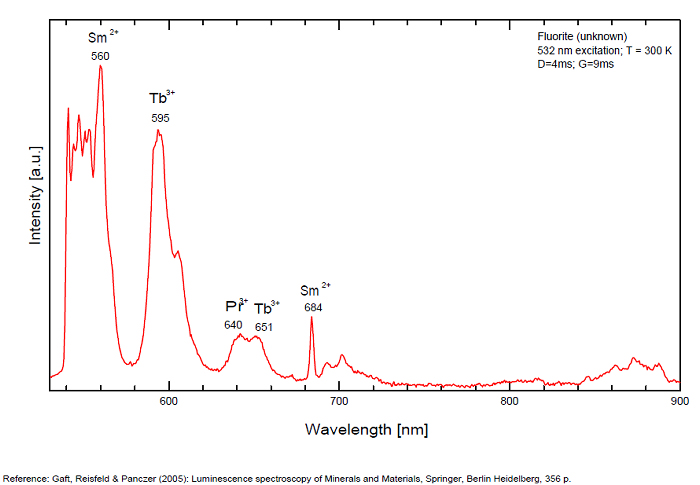
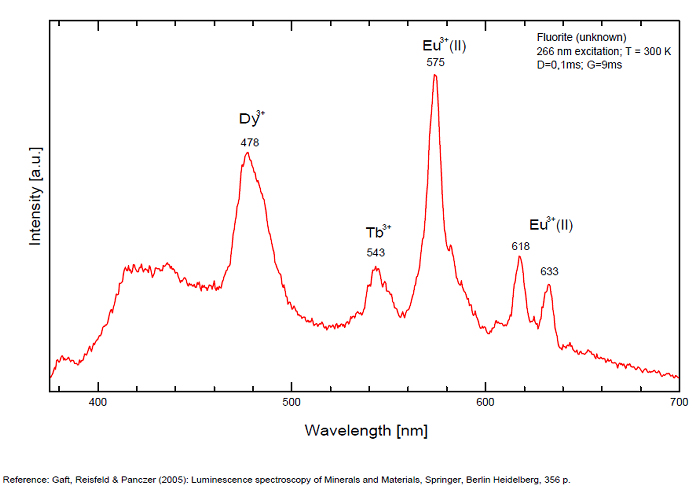
Violet fluo associated with Eu2+ (420-423 nm) with peaks at 320 and 340nm (Ce3+), decay time of Eu2+ is 600–800 ns λex = 415 nm is most suitable for measuring the Ho3+ emission beside the Er3+. Fluorite was one of the first mineral substances being investigated by George G. Stokes in 1852, hence the name fluorescence given at the phenomenon. Fluorite is a reservoir for many of the rare earth elements. As early as 1881, it was pointed out that Cerium was present in fluorite. In 1906, Urbain studied the cathodoluminescence of fluorite and ascribed the cause of fluorescence to RRE (Yttrium, praeseodymium, samarium, dysprosium, europium, terbium and also gadolinium and Ytterbium in chlorophane); Morse (1907), Tanaka (1924), Nichols (1928) and F. G. Wick also investigated the cause of luminescence of fluorite. The fusion of fluorescent fluorite (1100-1200°C) changes the spectrum markedly. Often the blue luminescence spectrum is changed to a more orange-red, showing sharp lines typical of RRE. De Ment makes 2 groups in the luminescent fluorites: group 1: single band in the UV at 300nm and 3 bands in the visible at 475, 508 and 578nm Radiation induced MA centers’ charge stabilized by Na+–Ca2+ is responsible of emission at 750nm and O2−–F− at 690 nm) (Tarashchan 1978, Gaft and Al. 2020).
yellow fluo associated with Dy3+;
M-center: large pic at 720 nm;
Lifetime: 2μs ( @420nm);
group 2: two bands in UV at 280 and 300nm and strong peaks at 475, 510, 536, 548 and 578nm in the visible.
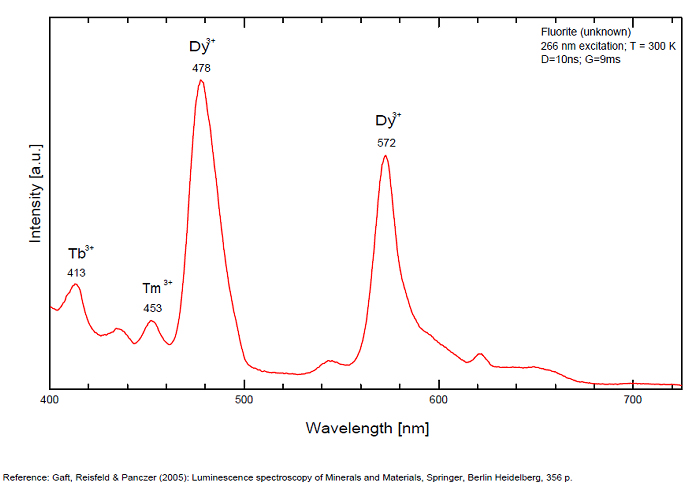
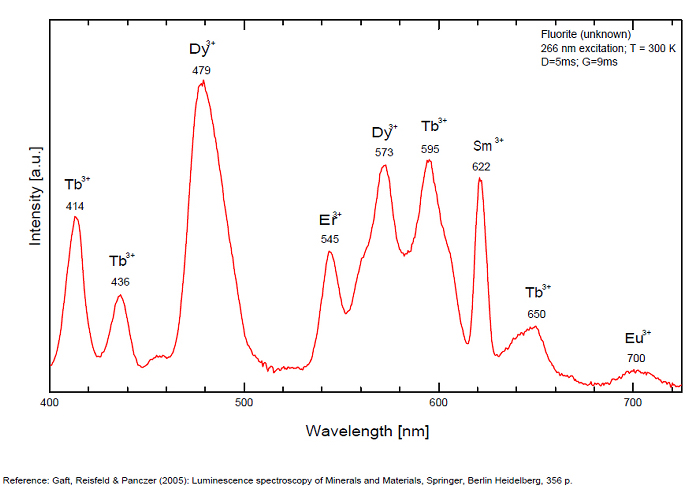
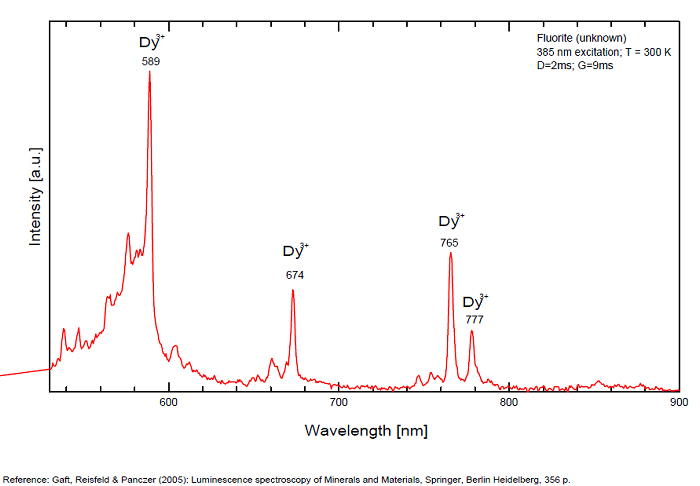
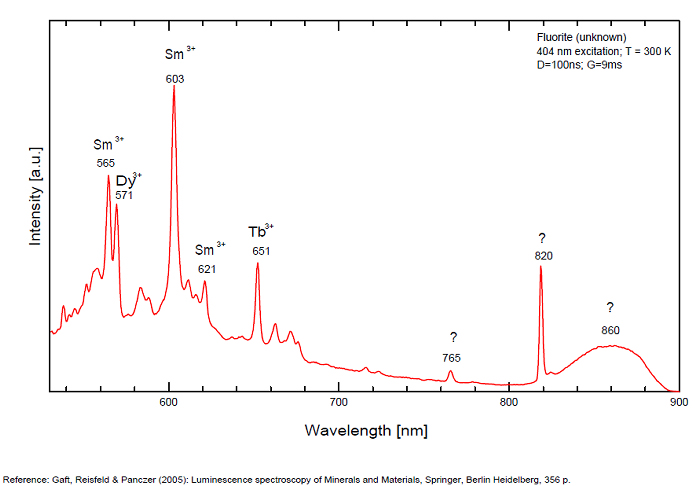
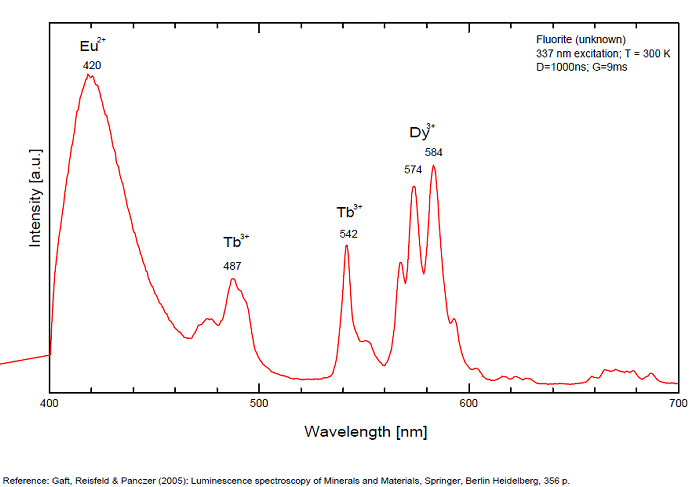
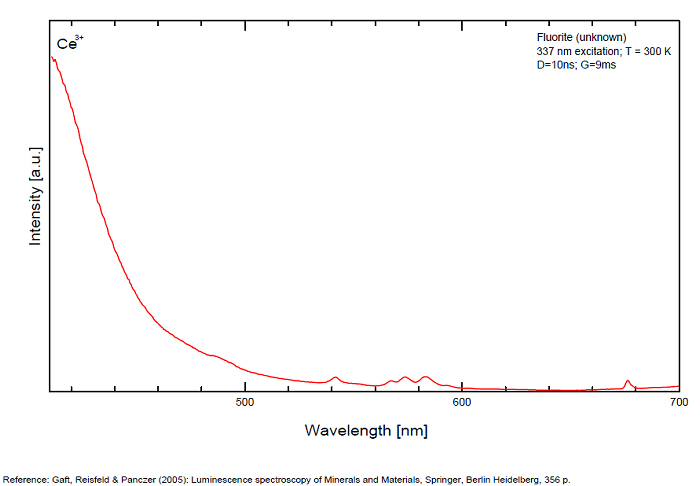
FONTANITECa(UO2)3(CO3)2O2 6H2O
Activator(s):
(UO2)2+ (ion Uranyle) intrinsèque ,
Other activators:
(UO2)2+ (ion Uranyle) intrinsèque ,
Peaks in the spectrum (nm):
(UO2)2+ : 549,5nm
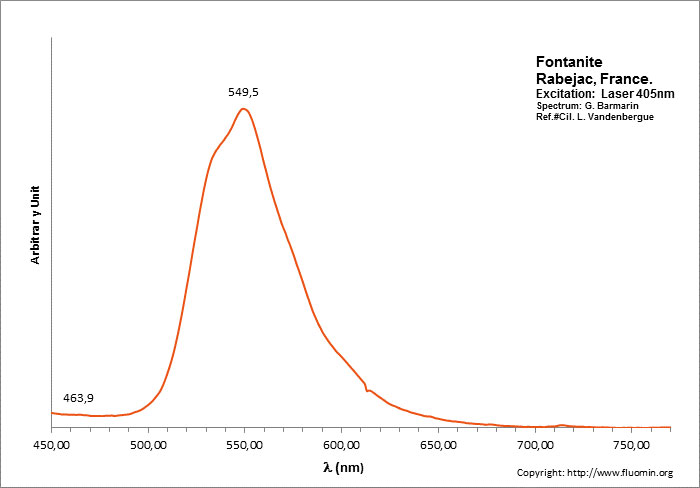
Excitation: laser 405nm. Col. L. Vandenberghe; Spectre: G. Barmarin
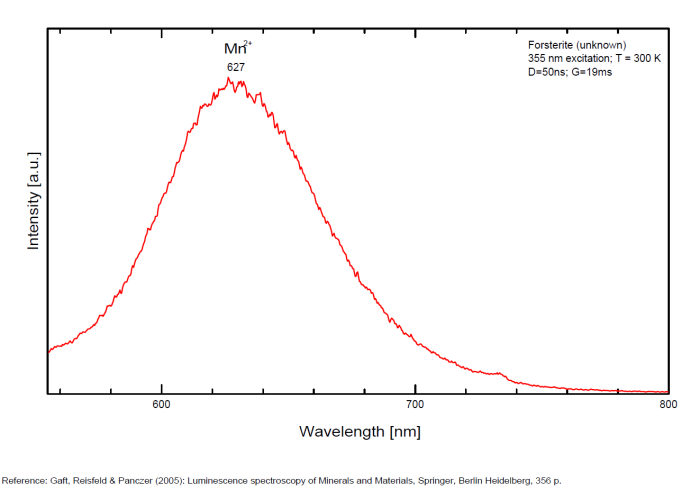
FORSTERITEMg2SiO4
Activator(s):
Mn2+ ,
Other activators:
Cr3+, Fe3+, O*,
Peaks in the spectrum (nm):
Mn2+ repl. Mg2+ : Broad band peaking at 627-660nm (decay time: 29ms) Cr3+ repl. Mg2+ (?) : 683, 693nm Fe3+ repl. Si4+ : 710nm O*Si around SiO4 : 420, 440nm O*Al (Al3+ - Si4+ : 470nm
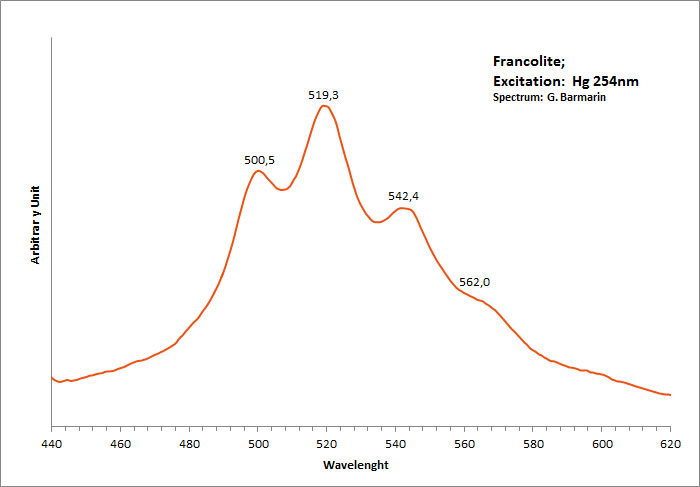
francolite
Activator(s):
(UO2)2+ (ion Uranyle) en impureté,
Peaks in the spectrum (nm):
400 nm, 463 nm, 490 nm, 527 nm,563 nm UO22+ : 500, 519, 542, 562nm (sample in collection)
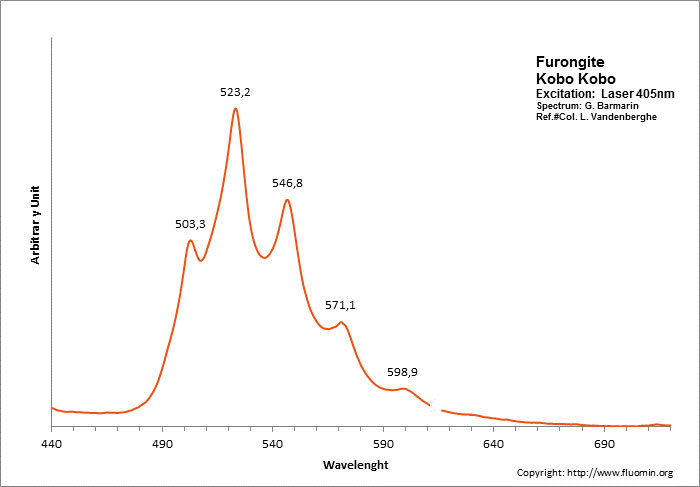
FURONGITEAl2(UO2)(PO4)2(OH)2 8H2O
Activator(s):
(UO2)2+ (ion Uranyle) intrinsèque ,
Peaks in the spectrum (nm):
(UO2)2+ : 503, 523, 547, 571, 599nm
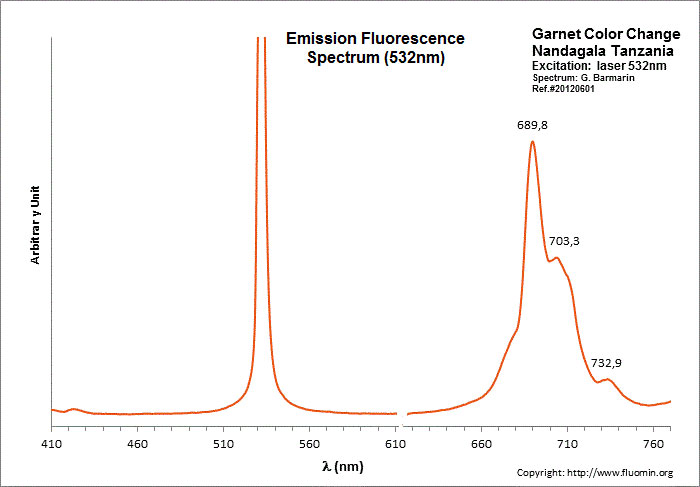
garnet
Activator(s):
Mn2+ ,
Other activators:
V2+, Nd3+,
Peaks in the spectrum (nm):
Nd3+ : 462, 476, 482, 501nm Mn2+ : band peaking at 590nm (60nm half-width) V2+ : 717nm
The specified directory does not exist.
GROSSULARCa3Al2(SiO4)3
Activator(s):
Cr3+,
Other activators:
Mn4+, Mn2+ , V2+,
Peaks in the spectrum (nm):
Broad band peaking at 611nm, peak at 489nm and 713nm (échantillon en collection) Cr3+ : Lines at 690, 694, 698 and 707nm Mn2+ substituting to Ca2+ : band around 590 -605 nm Mn3+ : broad band at 653nm Mn4+ : broad band at 670nm V2+ : peak at 717 nm (Gaft)
Comments on spectra and activators:
The color is identical to that of red-fluorescing ruby corundum or spinel. As with those minerals, chromium in place of aluminum is the likely activator. Other activator: V2+ (717 nm), Nd3+ (462, 476, 482,501 nm) and possibly Mn2+ band around 605-610 nm (decay time: several ms). Natural grossular luminescence was studied by continuous wave (CW) luminescence technique and has been connected with Cr3+ centers, which substitutes for Al3+ and occupies a site with distorted octahedral symmetry (which in fact is trigonal C3i). Trivalent chromium in grossular occupies high crystal field position. The luminescence spectra of Cr3+ in grossular at room temperature contain a strong broad band peaking at 720 nm and three sharp lines centered at 697, 700 and 701 nm. Excitation by CW laser with 785 nm revealed IR luminescence lines which evidently may be ascribed to trivalent REE, such as Pr, Ho, Nd, Er and Yb (Gaft).
The specified directory does not exist.
GYPSUMCaSO4 2H2O
Activator(s):
ST (Singlet-triplet)-Matière organique en impureté,
Other activators:
(UO2)2+ (ion Uranyle) en impureté,
Peaks in the spectrum (nm):
ST : 440-630nm 494nm, 510nm, 544nm, 601nm (Hourglass figure, Canada) UO22+ : 469, 486, 507, 530, 554nm (sample from Morrocco)
Comments on spectra and activators:
Typical uranyl spectrum when green luminescence is present.
Greenish-white and bluish fluorescence most probably due to organic impurities.
The specified directory does not exist.
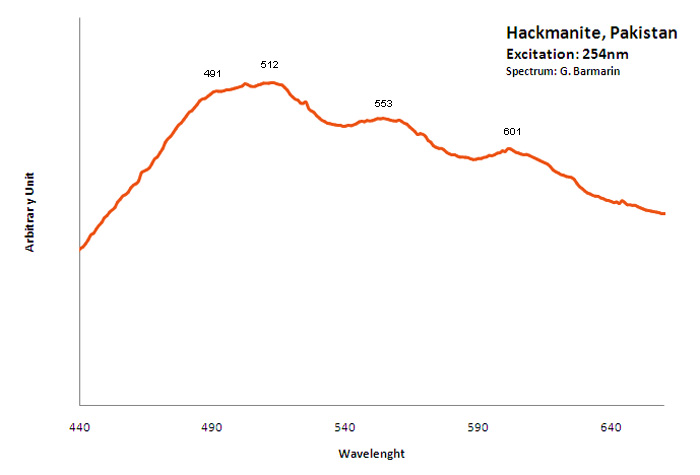
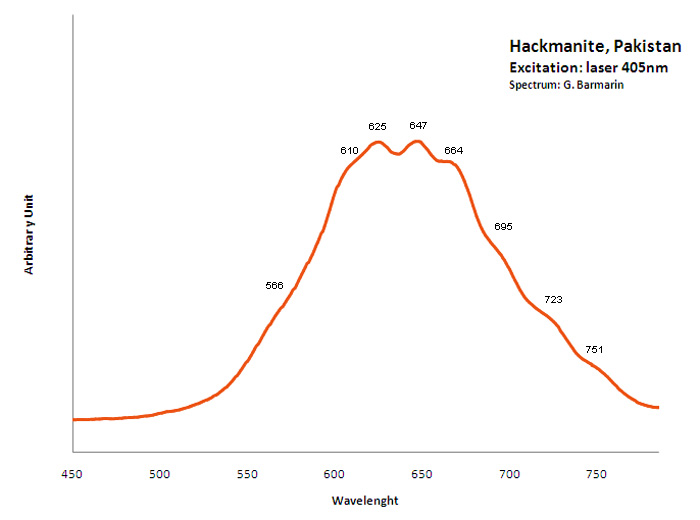
hackmanite
Activator(s):
S2-,
Peaks in the spectrum (nm):
S2- : (566),(610), 625, 647, 664, (695), (723), (751nm)
Comments on spectra and activators:
O. Ivan Lee investigated what he calls the reversible photosensitivity of hackmanite from Bancroft (Ontario) and his response to different UV sources as early as 1936. He presented the phenomenon for the 50th Anniversary Celebration Banquet of the New York Mineralogical Club, in November 18, 1936 at the American Museum of Natural History. It seems that it was the first observation and the first public announcement and publication (American Mineralogist vol 21) about photochromism (tenebrescence) in mineralogy. Chemical analyses revealed that the mineral contains a certain amount of sulfur as a substitute for chlorine in the crystal structure. The FTIR spectra of hackmanite showed that the samples contain water. The stretching vibration peak of water of crystallization (H2O) occurs at 3438 cm-1 and the bending peak is at 1623 cm-1. Its tenebrescence is caused by hole color centers which are contributed to the presence of sulfur (S2-)) and to some negatively charged chlorine atoms being missing in the crystal structure of hackmanite. (source: http://www.geology.com.cn/Geology-Journals/article-35765.html) Crystals of Hackmanite of Koksha Valley in Afghanistan are usually found in a matrix constituted by non-fluorescing Winchite and/or marble. Synthetic sodalites containing sulfur and showing considerable photochromic activity have been investigated by ESR. The center responsible for the color has been shown to be an electron trapped at a chlorine vacancy. The origin of the electron which is reversibly transferred during the processes of coloration and bleaching is believed to be the ion S2-). (see William G. Hodgson, Jacob S. Brinen, and Emil F. Williams, Electron Spin Resonance Investigation of Photochromic Sodalites, The Journal of Chemical Physics 47, 3719 , 1967)
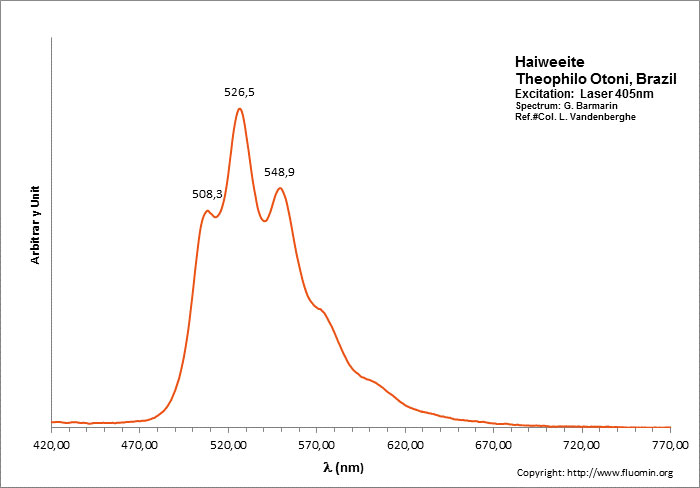
HAIWEEITECa[(UO2)2Si5O12(OH)2] 3H2O
Activator(s):
(UO2)2+ (ion Uranyle) intrinsèque ,
Peaks in the spectrum (nm):
(UO2)2+ : 508, 526, 549nm
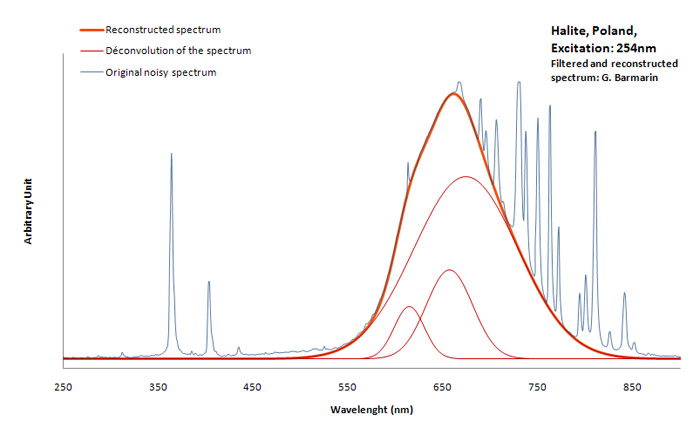
HALITENaCl
Activator(s):
Mn2+ ,
Peaks in the spectrum (nm):
Mn2+ repl. Na+ with Pb as co-activator : 590nm (band) Mn2+ interstition : 630 nm (band)
Comments on spectra and activators:
Activator for red: Mn2+ with Pb2+ as co-activator. Color : more orange or more red depending of Mn concentration.
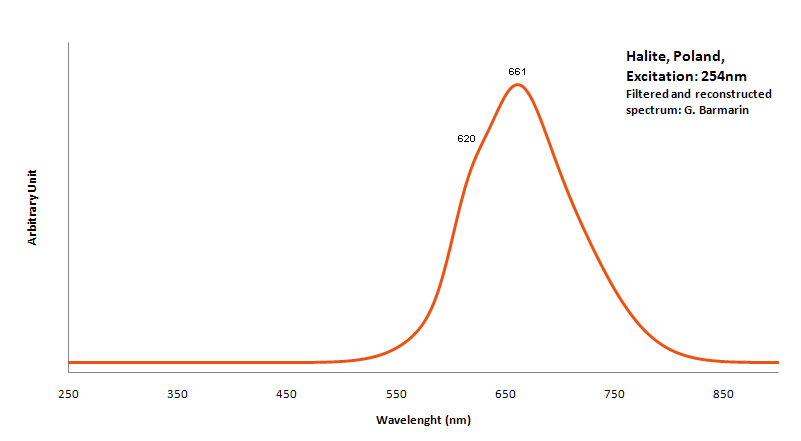
HALLOYSITEAl2Si2O5(OH)4 2H2O
Activator(s):
(UO2)2+ (ion Uranyle) en impureté,
Peaks in the spectrum (nm):
UO22+ : 499, 519, 542, 565nm
Comments on spectra and activators:
Typical spectrum of Uranyl Impurities
The specified directory does not exist.
HANKSITEKNa22(SO4)9(CO3)2Cl
Activator(s):
,
Peaks in the spectrum (nm):
490, 517, 553, 600, 650, 710nm
Comments on spectra and activators:
The luminescence of Hanksite was noticed by Kunz and Baskerville (1903) and has been studied by Melhase (1939).

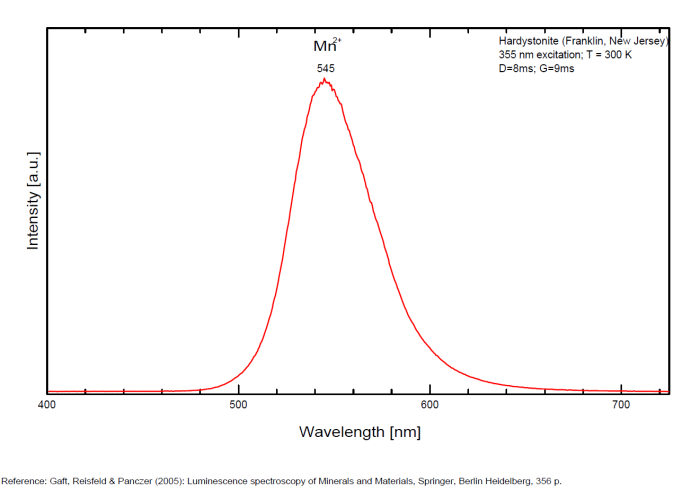
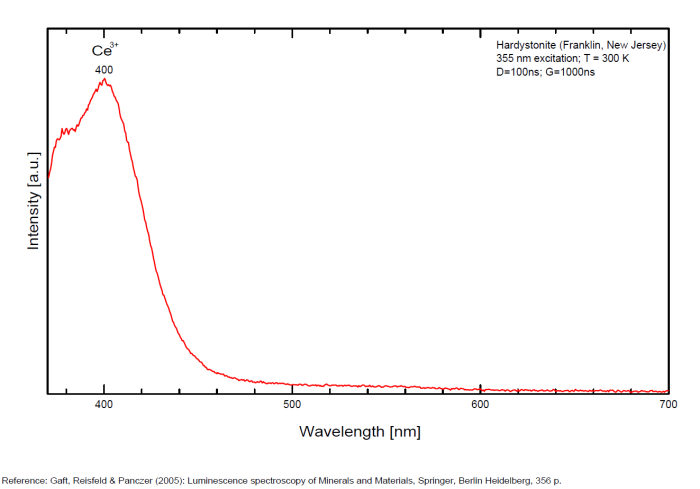
HARDYSTONITECa2ZnSi2O7
Activator(s):
Mn2+ ,
Other activators:
Pb2+, Ce3+, Dy3+, Gd3+, Tm3+,
Peaks in the spectrum (nm):
Pb2+: 355nm
Ce3+: 378nm
Ce3+: 400nm
Mn2+: 525nm
Mn2+: 545nm
Mn2+: 575nm
Gd3+ : 312nm
Dy3+ : 480, 575nm
Tm3+ : 452nm
Comments on spectra and activators:
Pb2+ : 355 nm
Mn2+ : 525 and 575nm
Ce3+ : 378, 400 nm
Tm3+, Dy3+
Decay time: several ms
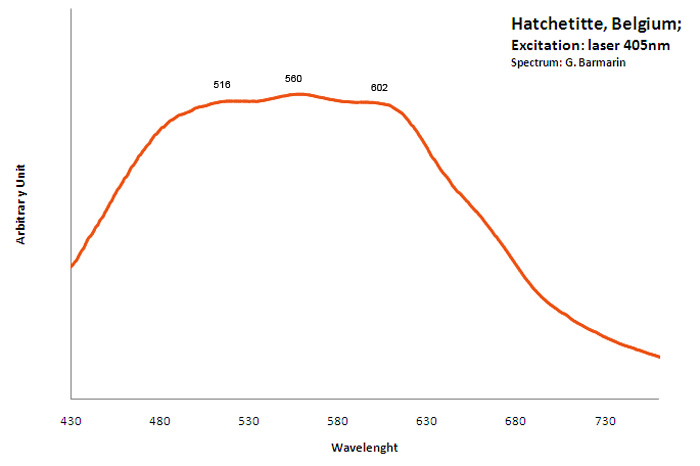
hatchettite
Activator(s):
Matière organique intrinsèque,
Peaks in the spectrum (nm):
Very Broad Band centered around 550nm;
HAUYNE(Na,Ca)4-8Al6Si6(O,S)24(SO4,Cl)1-2
Activator(s):
S2-,
Peaks in the spectrum (nm):
S2- repl.[SO4]2- : band centered around 680 nm with ondulations at: 516, 563, 580, 604, 621, 644, 666, 691, 715, 745nm
Comments on spectra and activators:
Form of the spectrum: typical wavelet of S2- upon a large band.
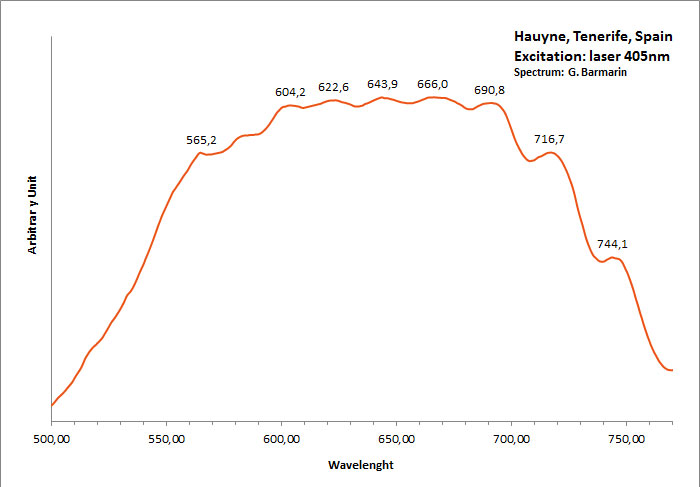
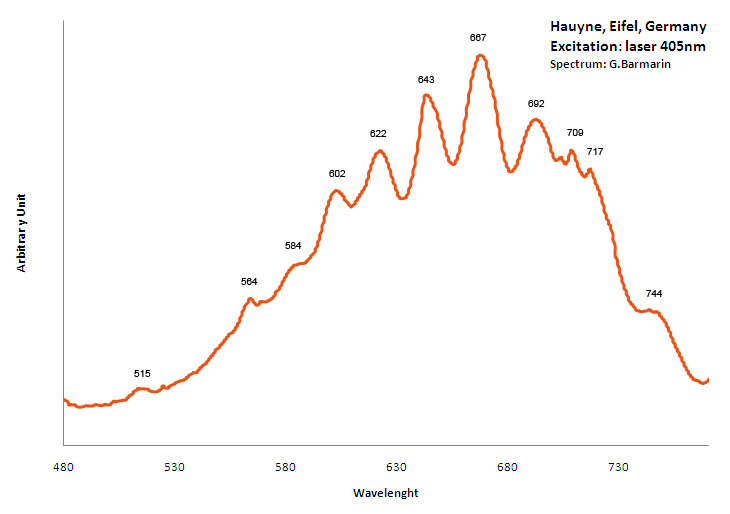
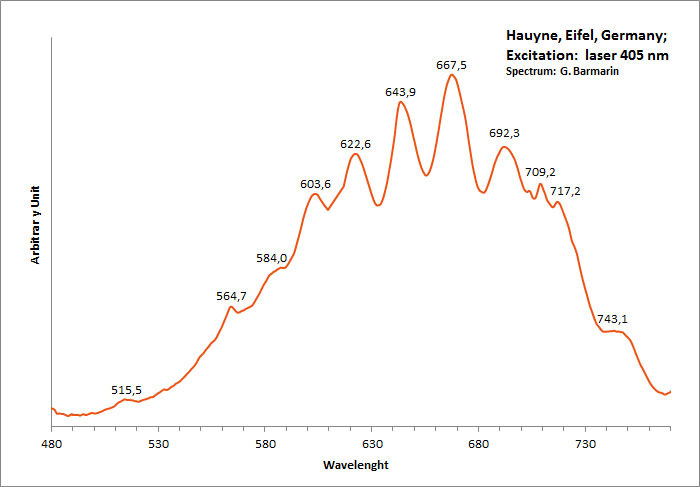
Excitation: laser 405nm. Col. G. Barmarin; Spectre: G. Barmarin
HAYNESITE(UO2)3(Se+4O3)2(OH)2 5H2O
Activator(s):
(UO2)2+ (ion Uranyle) intrinsèque ,
Peaks in the spectrum (nm):
(UO2)2+ : 522, 541, 563, 591nm
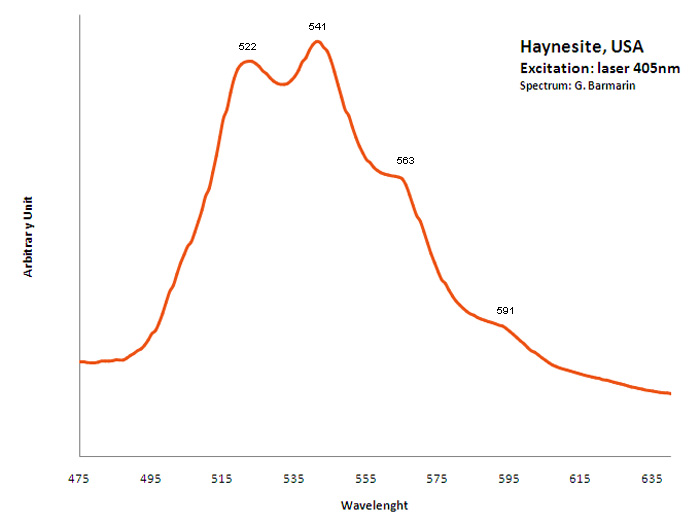
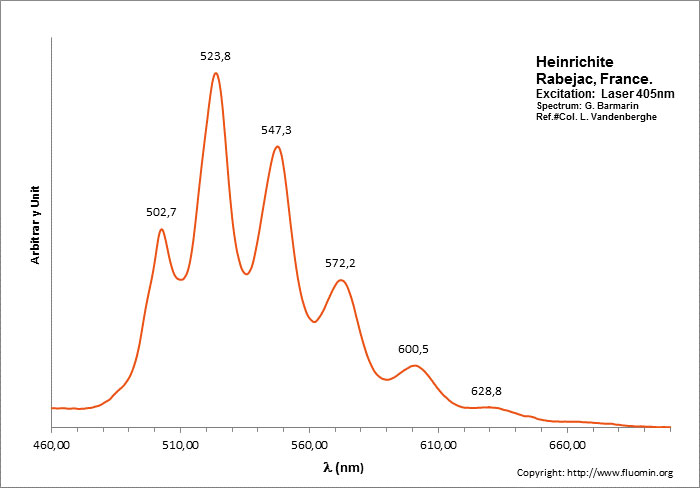
HEINRICHITEBa(UO2)2(AsO4)2 10-12 H2O
Activator(s):
(UO2)2+ (ion Uranyle) intrinsèque ,
Peaks in the spectrum (nm):
(UO2)2+ : 495.0, 505.8, 528.4, 551.4, 577.7, 604.6nm
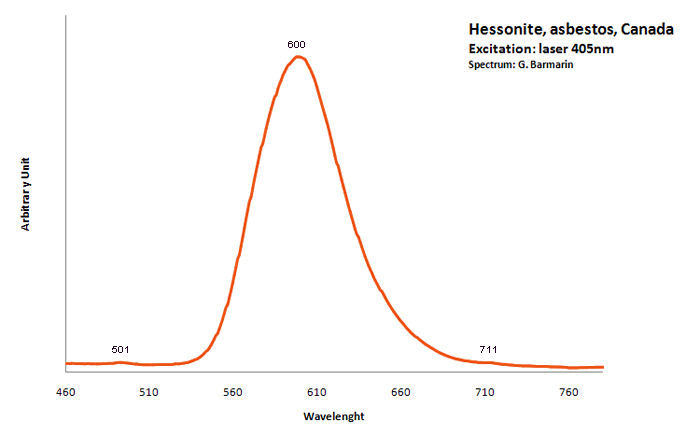
hessonite
Activator(s):
,
Other activators:
Mn2+ ,
Peaks in the spectrum (nm):
Mn2+ : Broad band peaking at 600nm (70nm half-width) ; peaks at 501nm and 711 nm ;
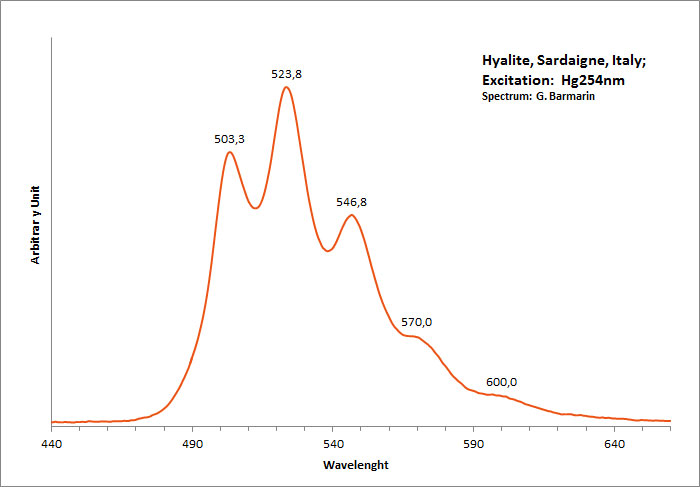
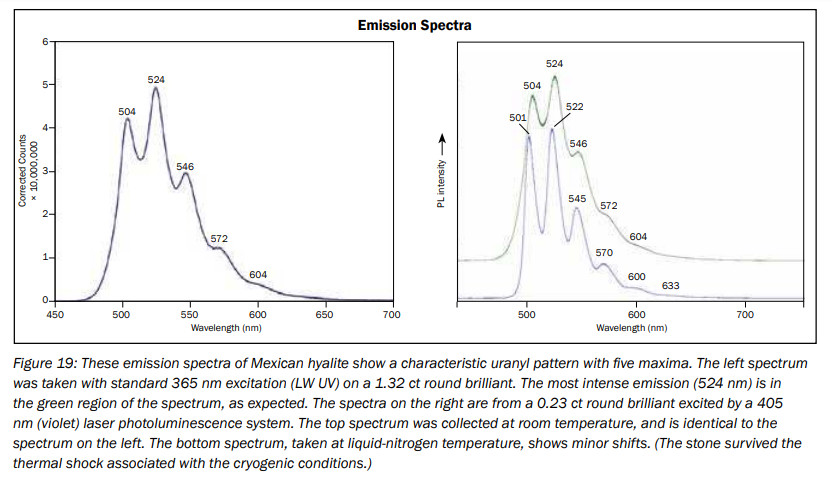
hyalite
Activator(s):
(UO2)2+ (ion Uranyle) en impureté,
Peaks in the spectrum (nm):
UO22+ : 503, 524, 547, 570, 600nm
Comments on spectra and activators:
Typical spectrum of Uranyl impurities.
HYDROBORACITECaMgB6O8(OH)6 3H2O
Activator(s):
,
Peaks in the spectrum (nm):
Broad band with events at: 530, 568, 601, 650nm

hydrocarbon
Activator(s):
ST (Singlet-triplet)-Matière organique en impureté,
Peaks in the spectrum (nm):
490nm, 512nm, 552nm, 596nm
The specified directory does not exist.
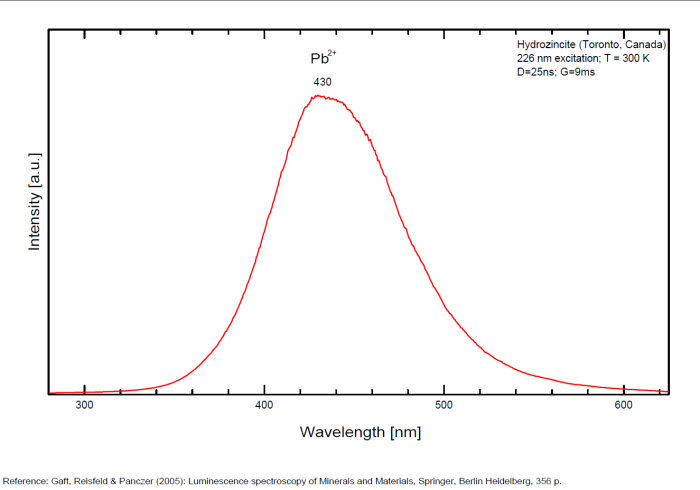
HYDROZINCITEZn5(CO3)2(OH)6
Activator(s):
Pb2+,
Peaks in the spectrum (nm):
Pb2+ replacing Zn2+ in octahedral coordination (Gaft): Broad band peaking at +/- 430-450 nm
Comments on spectra and activators:
Lifetime: 1μs
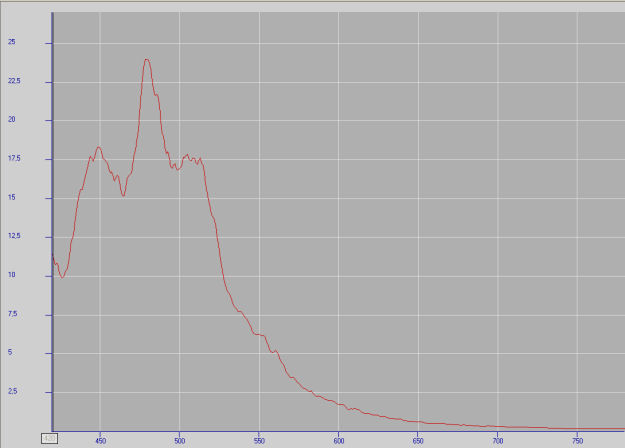
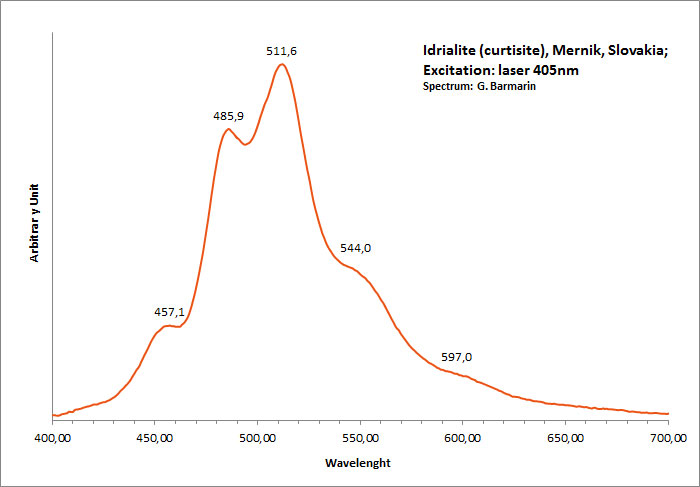
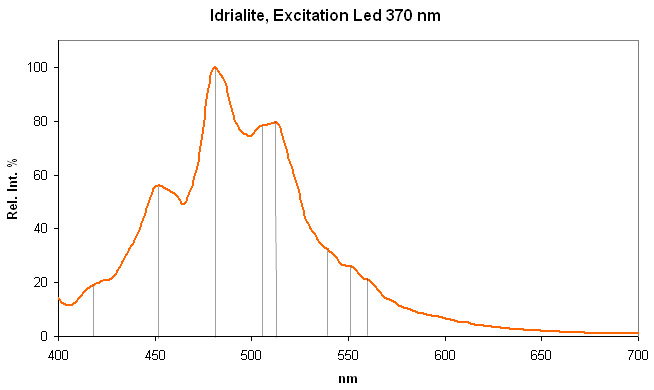
IDRIALITEC22H14
Activator(s):
Matière organique intrinsèque,
Peaks in the spectrum (nm):
Peaks at: 454nm, 482nm, 512nm, 544nm, 597nm ;
Comments on spectra and activators:
Blue luminescence due to the two linearly annelated benzene rings characteristic of aromatic compound (singlet-singlet electron transition within the benzene rings.Spectral lines (cm-1): 250, 235, 222, 208;
ivory
Activator(s):
Matière organique intrinsèque,
Peaks in the spectrum (nm):
520nm, 554nm, 592nm
The specified directory does not exist.
JOHANNITECu(UO2)2(SO4)2(OH)2 8H2O
Activator(s):
(UO2)2+ (ion Uranyle) intrinsèque ,
Peaks in the spectrum (nm):
501.3, 525.8, 548.5, 578.0 +/-3nm
KAMOTOITE-(Y)Y2U4+6(CO3)3O12 14,5H2O
Activator(s):
(UO2)2+ (ion Uranyle) intrinsèque ,
Peaks in the spectrum (nm):
(UO2)2+ : 519, 535nm
/spectres/KAMOTOITE-(Y)spectre.jpg)
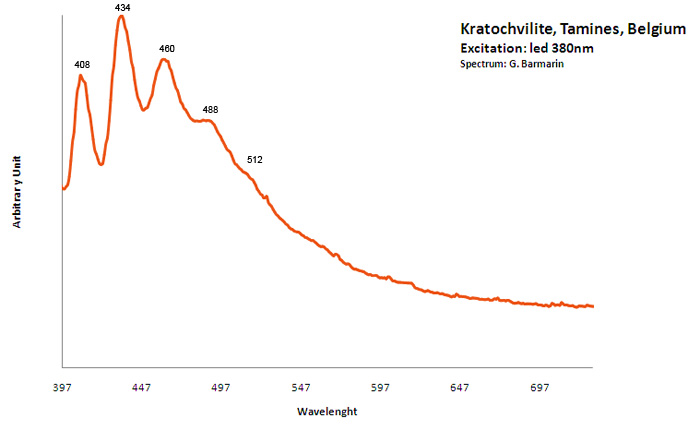
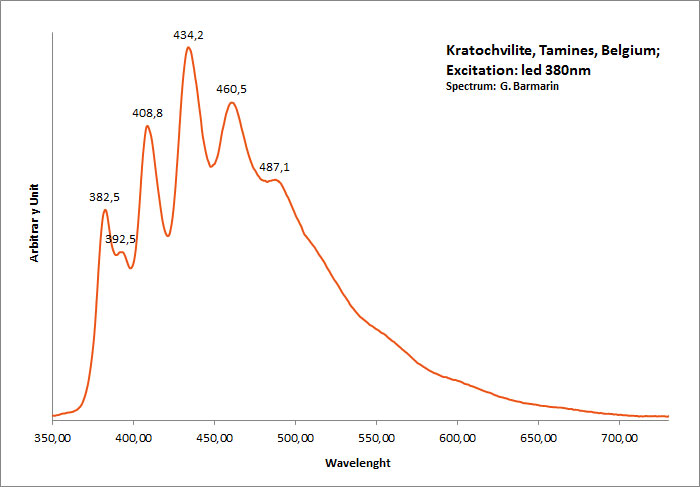
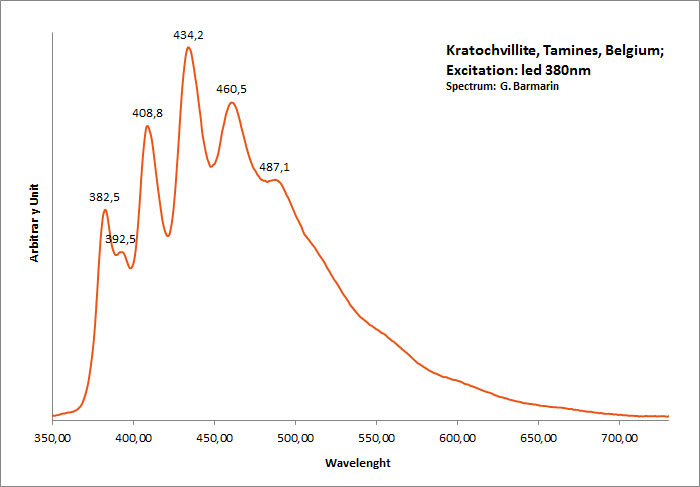
KRATOCHVILITEC13H10
Activator(s):
Matière organique intrinsèque,
Peaks in the spectrum (nm):
large band. max: 460nm, band at 572 nm / peaks at: 408nm, 434nm, 460nm, 488nm, 512nm
Comments on spectra and activators:
Organic compound formed in burning pyritic shale or coal fires. It is not clear whether kratochvillite is the chemical fluorene (C13H10) or anthracene (C14H10).
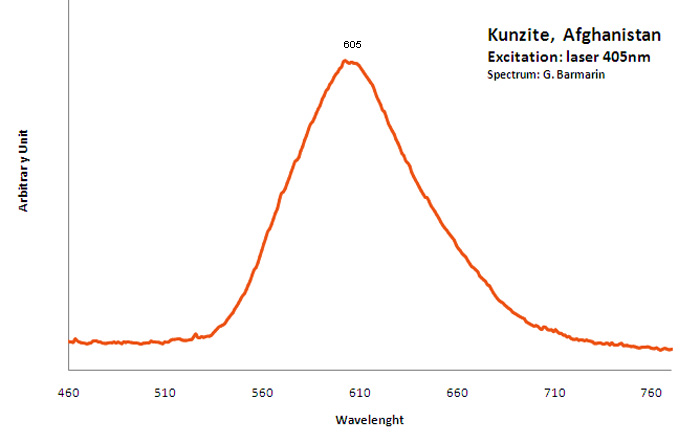
kunzite
Activator(s):
Mn2+ ,
Other activators:
Cr3+, TiO6,
Peaks in the spectrum (nm):
Large band at 430 nm, Mn2+ : large band peaking around 620 nm (Gorobets) large band peaking at 605nm
Comments on spectra and activators:
The phosphorescence of Kunzite was discovered by G. F. Kunz himself in 1903. Baskerville also studyed Kunzite luminescence in 1903. Extended studies were later made on the spectra of Kunzite by Pochettino and by Nichols who found that the fluorescence emitted light was polarized. Nichols also describe the fluorescence emitted by electron excitation as two bands : a strong broad band from 515nm to 690nm and a weak one extending from 420nm to 480nm. He also pointed that the band around 600nm have a strong phosphorescence and the other band have none. Nichols studied the thermoluminescence at 325°C and found that it was not polarized. The thermoluminescence ceased at 400°C. At 20°C the peak around 590nm is nearly symetric being somewhat steeper toward the violet. On cooling to -180°C, the fluorescence color becomes much redder to the eye but the spectrum show no resolution into narrow bands (DeMent 1949) Tanaka (1921-1932) demonstrated that the most important activating agents wich cause the luminescence of Kunzite is Manganese. However, he stated that Samarium and Ytterbium had a role as activator. In the Kunzite from Pala, Ca, USA, containing a few tenths of a percent of manganese, he found in the bright orange light emitted by fluorescence 12 manganese bands, 4 samarium bands and one thallium band.
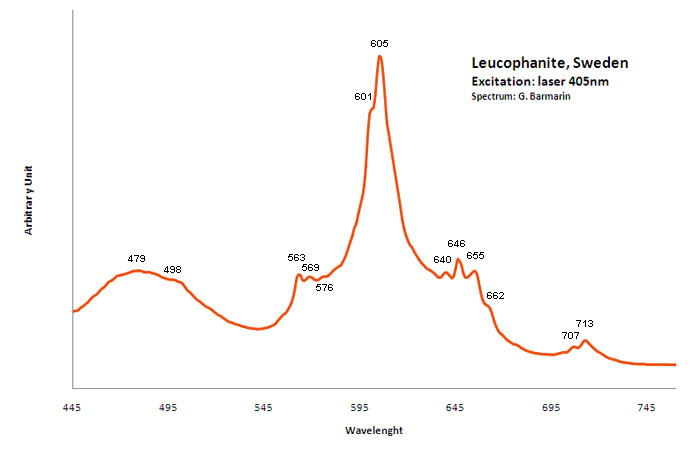
LEUCOPHANITE(Ca,RRE)CaNa2Be2Si4O12(F,O)2
Activator(s):
Ce3+,
Other activators:
Eu2+, Sm3+, Dy3+, Tb3+, Mn2+ , Nd3+,
Peaks in the spectrum (nm):
Ce3+ repl. Ca2+ : 375, 411, 450nm
Eu2+ : 466-470nm
Tb3+ : 546nm
Sm3+: 607nm
Dy3+: 475, 488, 576nm
Nd3+: 885, 1060nm
Mn2+: 610nm
Comments on spectra and activators:
Activators: a combinaison of RE elements - for exemple Ce3+ substituting to Ca (blue luminescence) and Mn (red luminescence) giving the magenta color (an orange flash typical of Mn(?) is seen on some samples). Cathodoluminescence: intense light-blue. The application of multiple forms of excitation (Friis et al. 2011) revealed that the UV-Blue emission in leucophanite and meliphanite consists of more than one emission center and is therefore more complex than previously thought. The most likely centers are defects related to the structure, e.g. in connection with the tetrahedral sites, and a Ce3+ centre. The difference in Na/Ca ratio between the two minerals make it possible for REE to substitute into two sites in meliphanite contrary, to just one in leucophanite. (Gaft)
Activators: Ce 3+, Eu 2+, Sm 3+, Dy 3+, Tb 3+, Nd 3+, Mn2+ substituting to Ca2+ (Gorobets in Gaft);
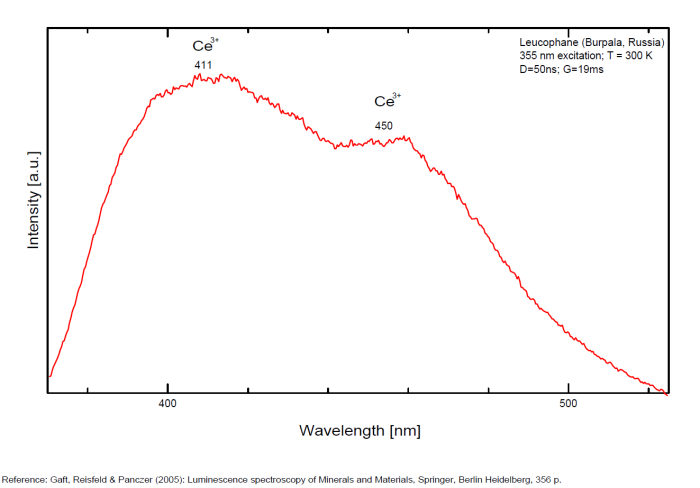
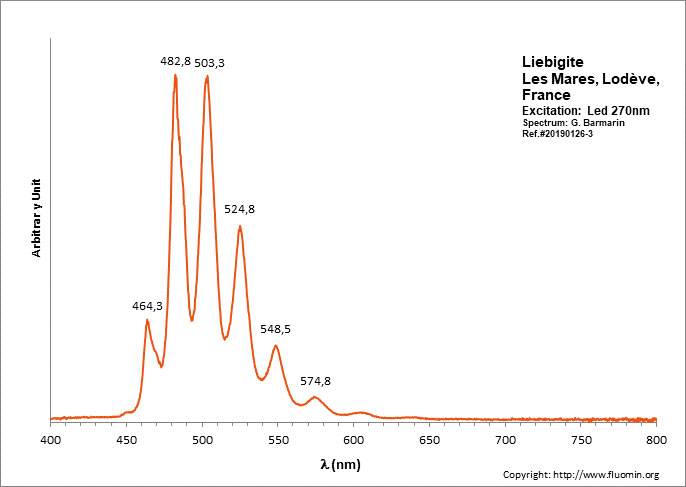
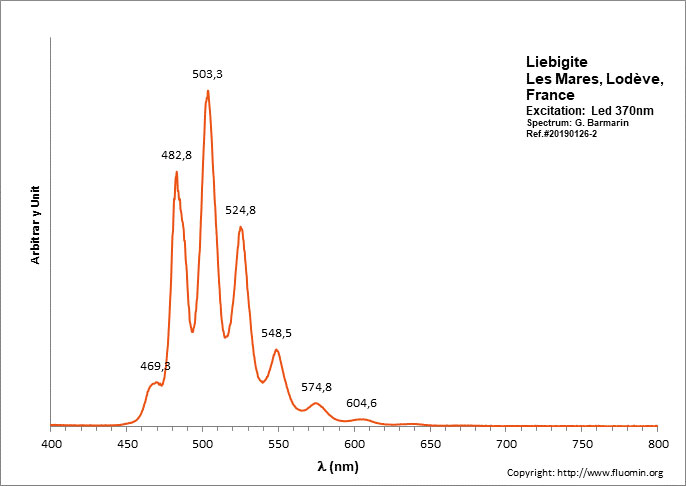
LIEBIGITECa2(UO2)(CO3)3 11H2O
Activator(s):
(UO2)2+ (ion Uranyle) intrinsèque ,
Peaks in the spectrum (nm):
(UO2)2+ : 464.3 (SW only), 469.3 (LW only), 482.8, 503.3, 524.8, 548.5, 574.8, 604.6, 638.5
Comments on spectra and activators:
Peak at 464.3 is SW only; In shortwave, peaks at 482.8 and 503.3 have approximately the same height giving a bluer apparence to the fluorescence color (estimated RGB color: 65, 255, 132). In Longwave, the peak at 503.3 dominates all other peaks giving a green fluorescence color (estimated RGB color: 65, 255, 100).






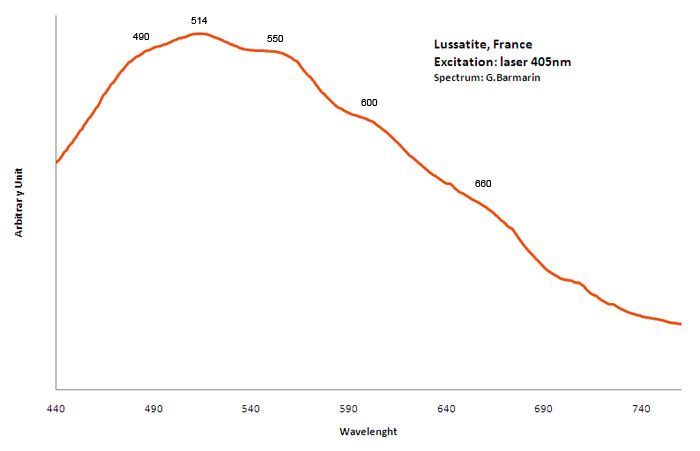
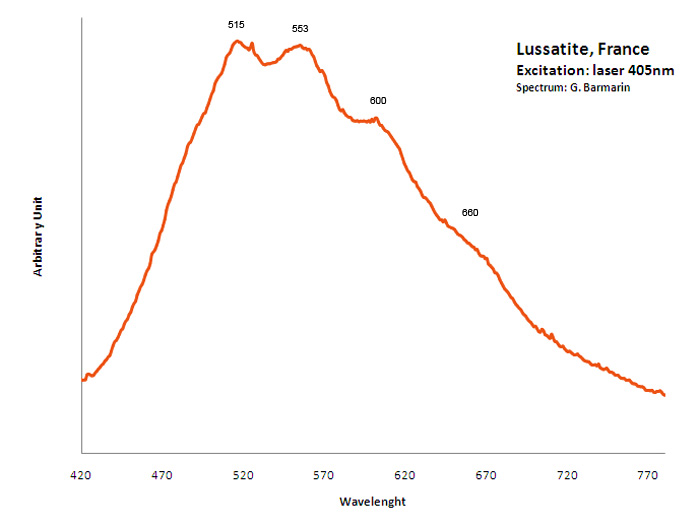
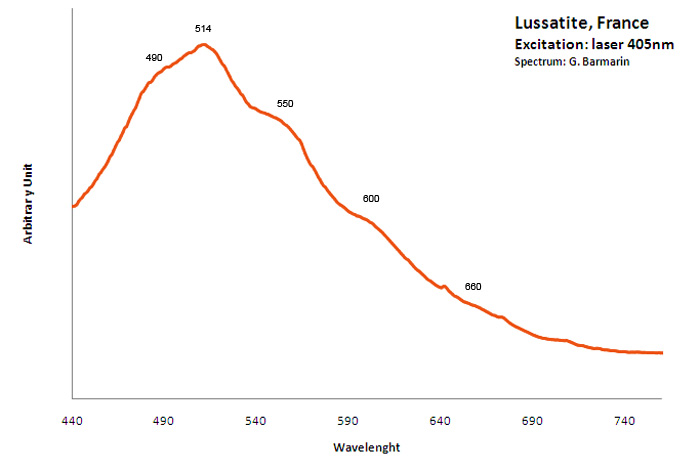
lussatite
Activator(s):
,
Peaks in the spectrum (nm):
490nm, 514nm, 550nm, 600nm, 660nm
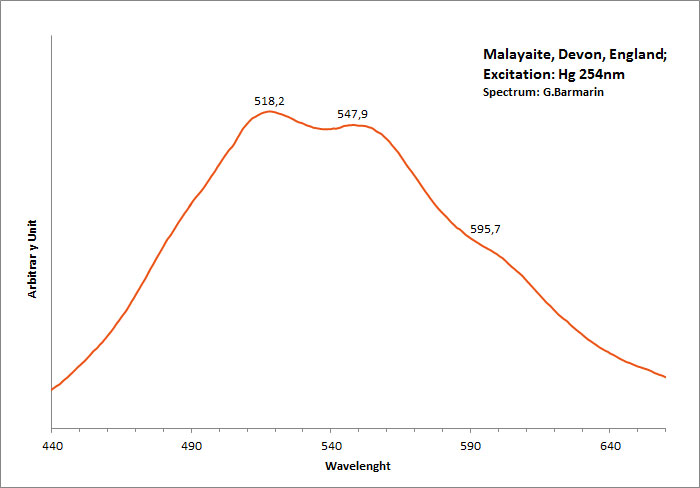
MALAYAITECaSnSiO5
Activator(s):
TiO6,
Other activators:
Sm3+, Mn2+ , Nd3+,
Peaks in the spectrum (nm):
TiO6? : band peaking at 550 nm Mn2+ : shoulder at +/- 630 nm (Gorobets)
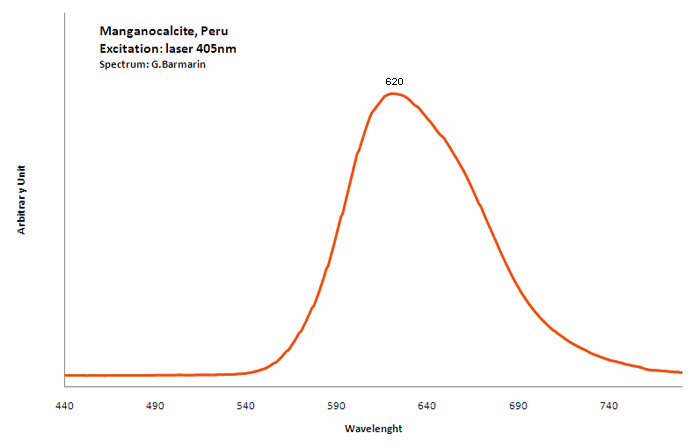
manganocalcite
Activator(s):
Mn2+ ,
Peaks in the spectrum (nm):
Broad band peaking at 620nm
META-ANKOLEITEK2(UO2)2(PO4)2 6H2O
Activator(s):
(UO2)2+ (ion Uranyle) intrinsèque ,
Peaks in the spectrum (nm):
505, 526, 550; 575, 615
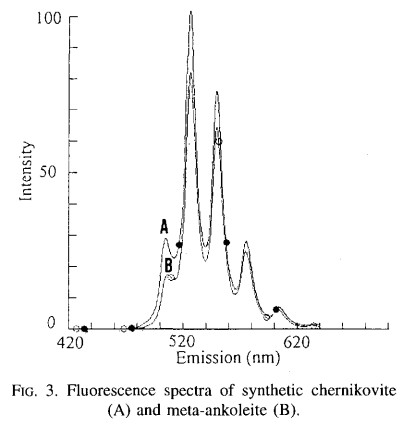
META-AUTUNITECa(UO2)2(PO4)2 2-6H2O
Activator(s):
(UO2)2+ (ion Uranyle) intrinsèque ,
Peaks in the spectrum (nm):
491.3, 501.8, 522.9, 546.9, 572.2, 591.7nm or 488, 501.6, 523.7, 547.2, 573.3, 600.2nm or 503nm, 524nm, 547nm, 572nm, 600nm, 631nm (échantillon en collection)
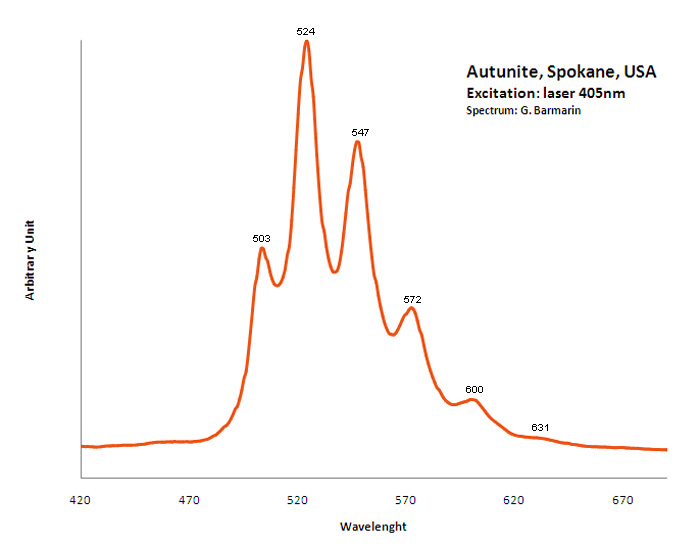
METAHEINRICHITEBa(UO2)2(AsO4)2 8H2O
Activator(s):
(UO2)2+ (ion Uranyle) intrinsèque ,
Peaks in the spectrum (nm):
(UO2)2+ : 492.4, 505.7, 527.9 (527), 551.4, 576.9 (575), 606.1 (603)
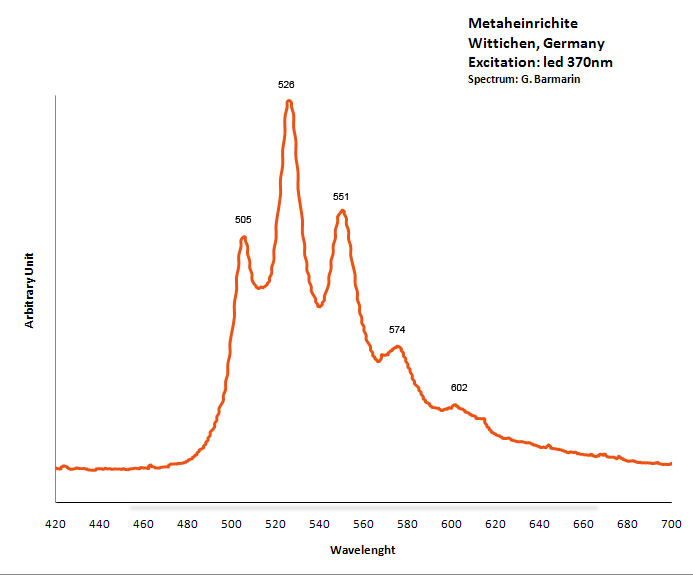
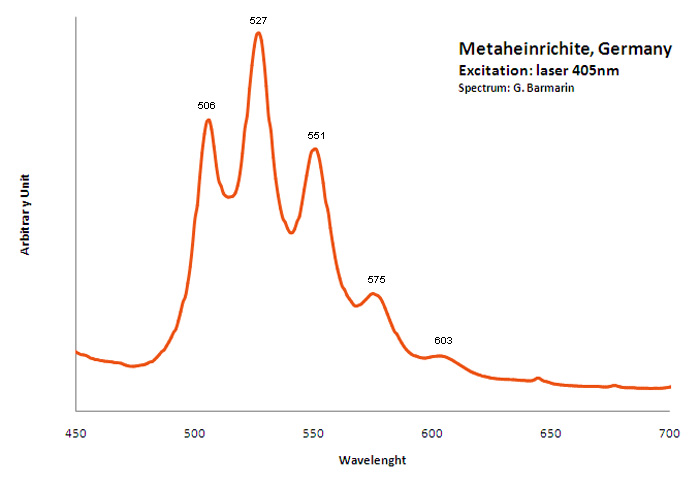
Excitation: laser 405nm. Col. G. Barmarin; Spectre: G. Barmarin
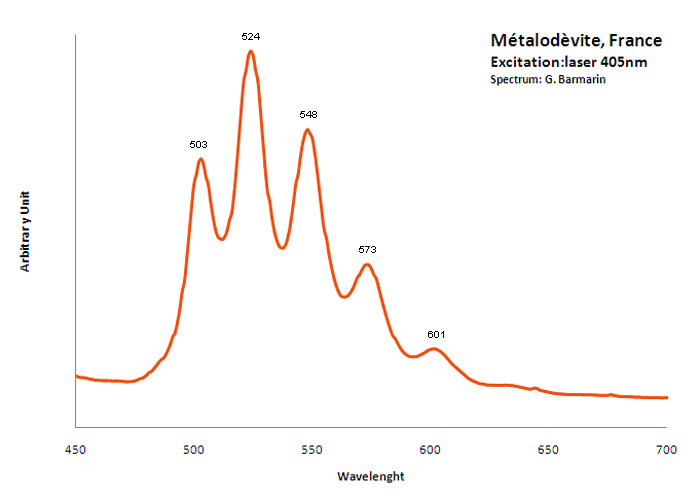
METALODEVITEZn(UO2)2(AsO4)2 10H2O
Activator(s):
(UO2)2+ (ion Uranyle) intrinsèque ,
Peaks in the spectrum (nm):
(UO2)2+ : 503nm, 524nm, 548nm, 573nm, 601nm
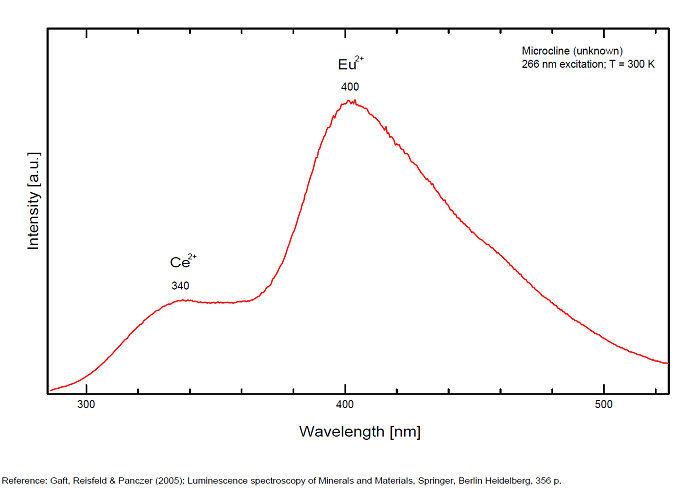
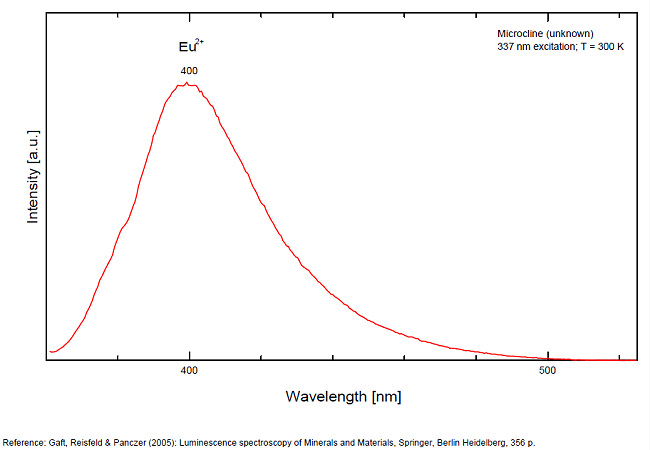
MICROCLINEKAlSi3O8
Activator(s):
Fe3+,
Other activators:
Eu2+, Ce3+, Eu3+,
Peaks in the spectrum (nm):
Ce3+: broad band peaking at 335nm
Eu3+: broad band peaking at 402
Eu2+: 395nm
Fe3+ repl. Al3+ or Si4+: band peaking at 700nm
Pb+ repl. K+: 900nm
Comments on spectra and activators:
Red fluorescence due to trivalent iron Fe3+. Cathodoluminescence: blue.
Blue fluorescence due to Ce3+ and Eu3+ (Gaft)
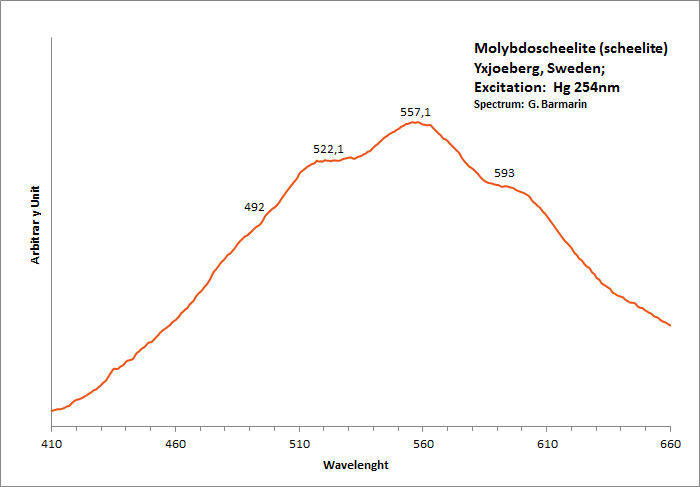
molybdoscheelite
Activator(s):
,
Peaks in the spectrum (nm):
(492nm), 522nm, 557nm, 593nm, (643nm)
MONAZITE-(Ce)(Ce,La,Nd,Th)PO4
Activator(s):
(UO2)2+ (ion Uranyle) en impureté,
Other activators:
Sm3+, Eu3+, Nd3+,
Peaks in the spectrum (nm):
(UO2)2+ : 506, 523 nm Laser induced luminescence lines corresponding to Sm3+, Eu3+, Nd3+: Sm3+ : lines at 607, 617, 642nm Nd3+ : weak lines at 792, 800, 806, strong lines at 863, 872, 877, 887, 897, 907nm
/spectres/MONAZITE-(Ce)spectre.jpg)
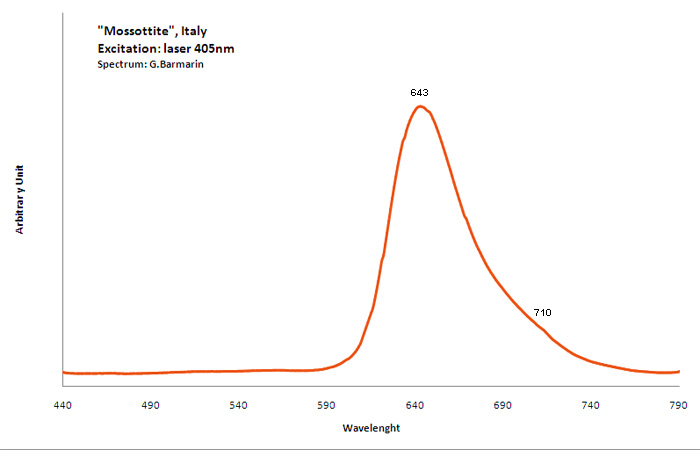
mossottite
Activator(s):
Mn2+ ,
Peaks in the spectrum (nm):
Broad band peaking at 643nm, wave at 710nm
Comments on spectra and activators:
The frequent occurrence of slight amounts of Strontium in aragonite was known during the time of Becquerel and he ascribed the luminescence to the presence of strontium. Later, Nichols confirmed the idea. Hence the name strontioaragonite for some specimen of very bright red fluorescing aragonite. Actually, strontium is not considered anymore as the activator responsable for the red fluorescence of aragonite and Mn2+ is considered as the principal activator of this red fluorescence.
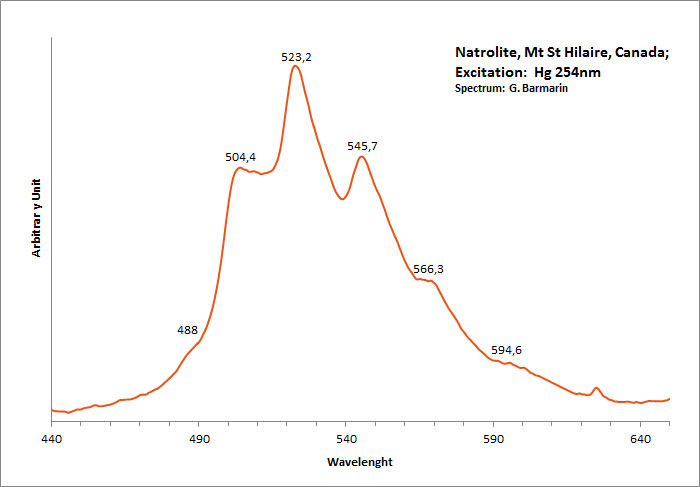
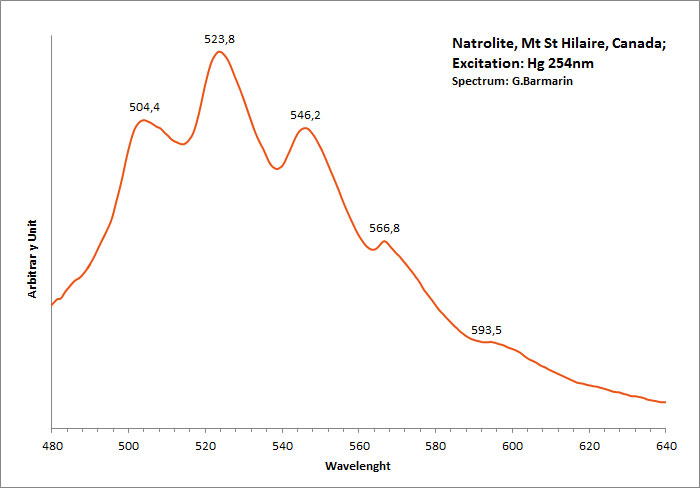
NATROLITENa2[Al2Si3O10] 2H2O
Activator(s):
(UO2)2+ (ion Uranyle) en impureté,
Peaks in the spectrum (nm):
(UO2)2+ : 488, 504 , 523-524, 545-546, 566, 593-594nm
Comments on spectra and activators:
Green luminescence due to Uranium in traces (typical spectrum of uranyl). Gunnel (1939) has studied the luminescence of natrolite from Oberschaffhauser, Germany (yellowish-white LW). Probably published in The Mineralogist at this time.
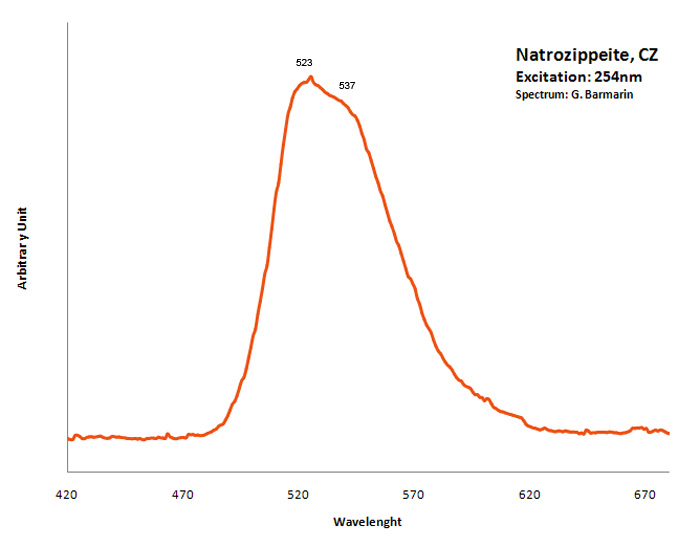
natrozippeite
Activator(s):
(UO2)2+ (ion Uranyle) intrinsèque ,
Peaks in the spectrum (nm):
Broad band around 532nm, probably the sum of two peaks at 523nm and 537nm
NESQUEHONITEMg(HCO3)(OH) 2H2O
Activator(s):
,
Other activators:
(UO2)2+ (ion Uranyle) en impureté,
Peaks in the spectrum (nm):
Very broad band with local max at 490, 517, 558, 600nm
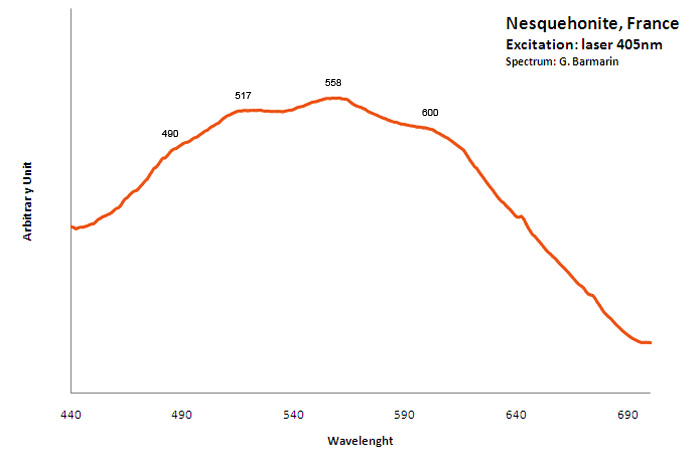
Excitation: laser 405nm. Col. G. Barmarin; Spectre: G. Barmarin
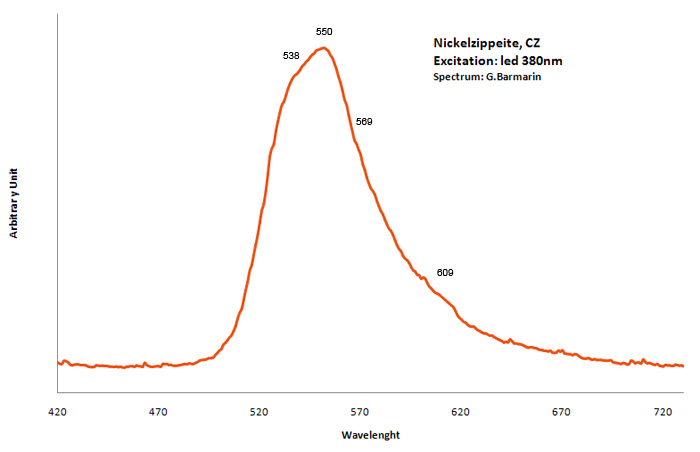
NICKEL-ZIPPEITENi2(UO2)6(SO4)3(OH)10 16H2O
Activator(s):
(UO2)2+ (ion Uranyle) intrinsèque ,
Peaks in the spectrum (nm):
Broad band around 548nm, probably the sum of two peaks at 538nm, 550nm, 569nm and 609nm
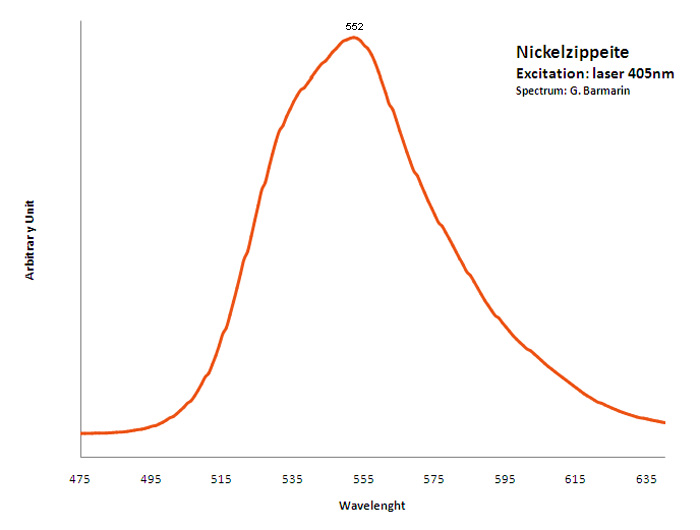
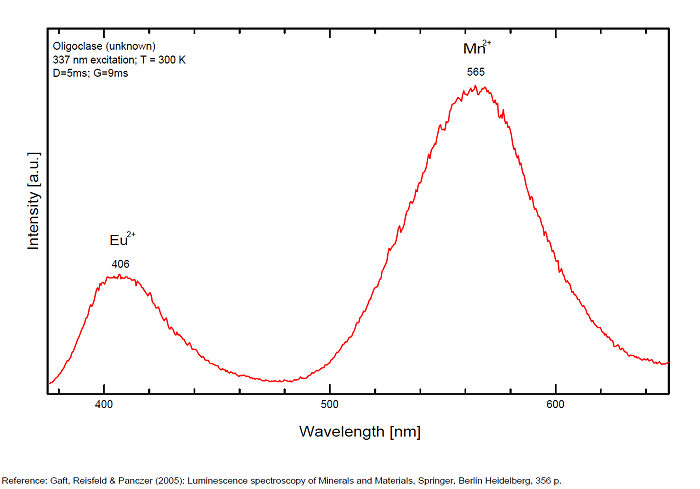
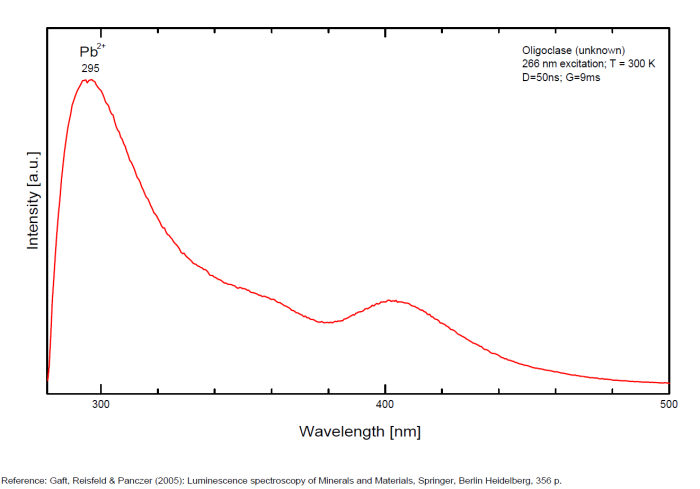
OLIGOCLASE(Na,Ca)(Si,Al)408
Activator(s):
Fe3+,
Other activators:
Pb2+, Mn2+ ,
Peaks in the spectrum (nm):
Pb2+ : broad band peaking at 296nm
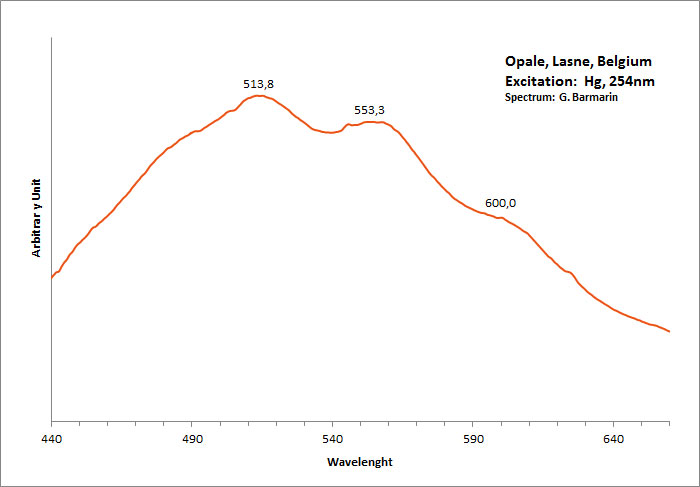
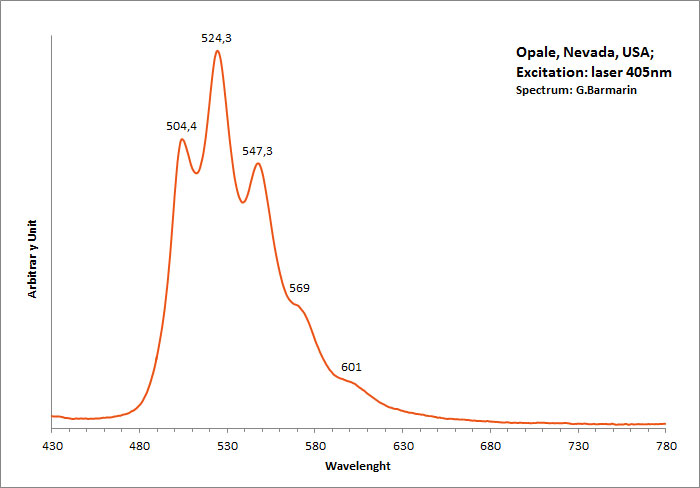
OPALESiO2 nH2O
Activator(s):
(UO2)2+ (ion Uranyle) en impureté,
Peaks in the spectrum (nm):
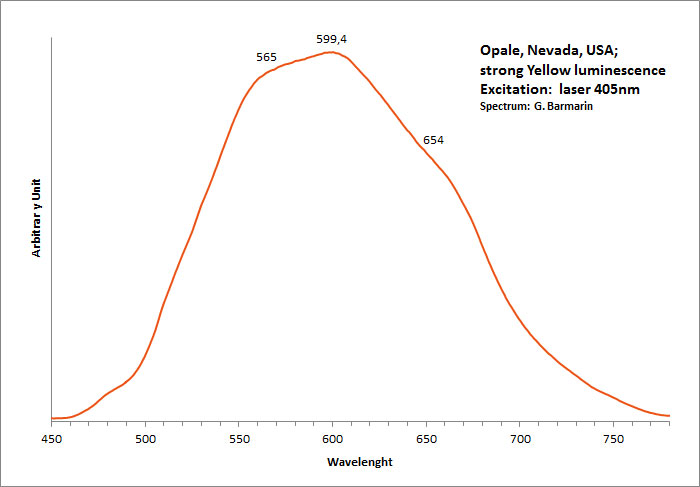
UO22+ : 504nm, 524nm, 547nm, 569nm, 601nm (opale Nevada, USA)
Comments on spectra and activators:
Green luminescence related to uranium impurities.
Georges O. Wild from Idar Oberstein in Germany found as early as 1947 that the greenish yellow fluorescence of opal from Virgin Valley, Nevada was due to uranium traces and being identical of the fluorescence of the synthetic gemstone Emerada (synthetic spinel).
Wild cites a weak band at 600 to 605nm, strong band at 570 to 578nm, strong band at 545 to 550nm, medium band at 522 to 527nm and weak band at 500 to 503nm.
Before Wild, DeMent has already studied the spectrum of opal s luminescence before Wild but not as precisely.
ozocerite/ (cire organique / organic wax)
Activator(s):
Matière organique intrinsèque,
Peaks in the spectrum (nm):
Broad band centered around 607nm with curve change at 525nm, 567nm, 607nm and 649nm

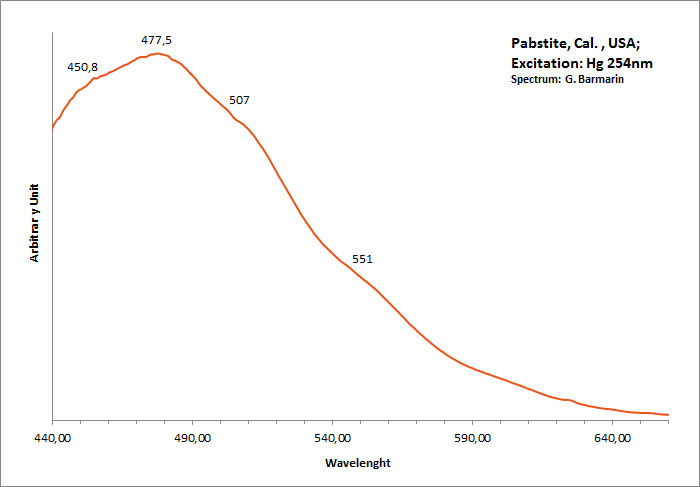
PABSTITEBa(Sn,Ti)Si3O9
Activator(s):
,
Peaks in the spectrum (nm):
Large band centered around 477nm with evants at 450nm, 477nm, 507nm, 551nm
Comments on spectra and activators:
Intrinsic luminescence similar to Benitoite.
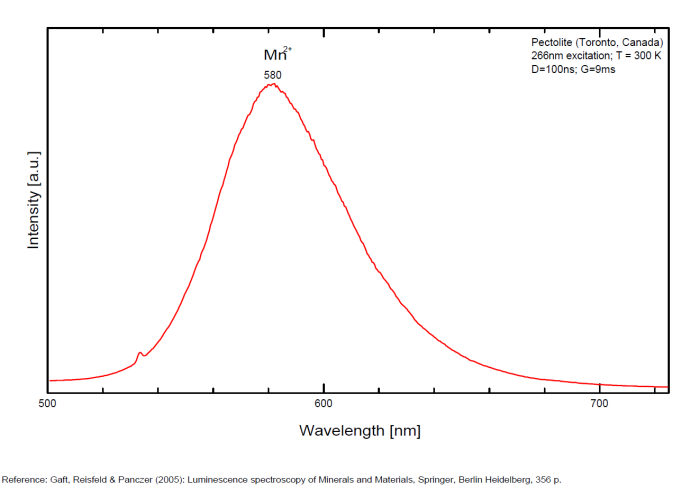
PECTOLITENaCa2Si3O8(OH)
Activator(s):
Mn2+ ,
Other activators:
Cr3+, Fe3+, Nd3+,
Peaks in the spectrum (nm):
Mn2+ replacing Ca2+ : 580 - 590 - 610nm ( Paterson NJ, USA sample, exc. 532nm) Fe3+ : 722nm (Asbestos Canada sample, exc. 532nm) Nd3+ : 867, 877, 881, 890, 918nm (Mt St Hilaire Canada sample, exc. 780nm) OH : 652nm (Diako, Sandare District, Mali sample, very sharp peak, exc. 532nm) (Gaft)
Comments on spectra and activators:
Activator: probably Mn2+ substituting to Ca2+ (see Gorobets)
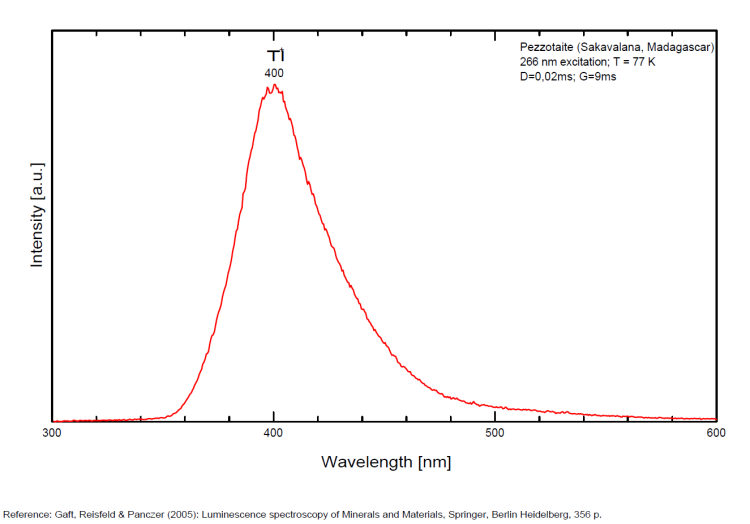
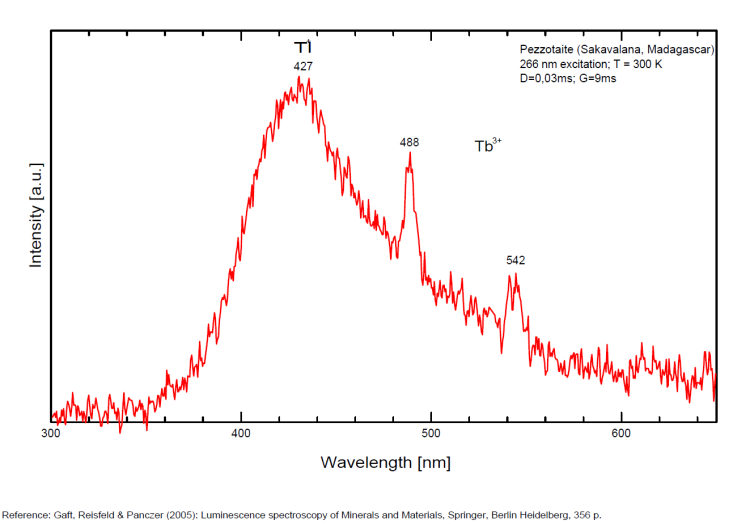
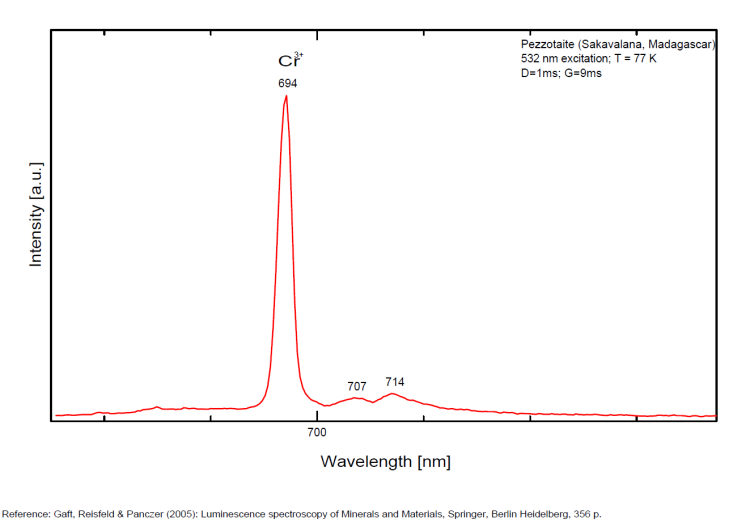
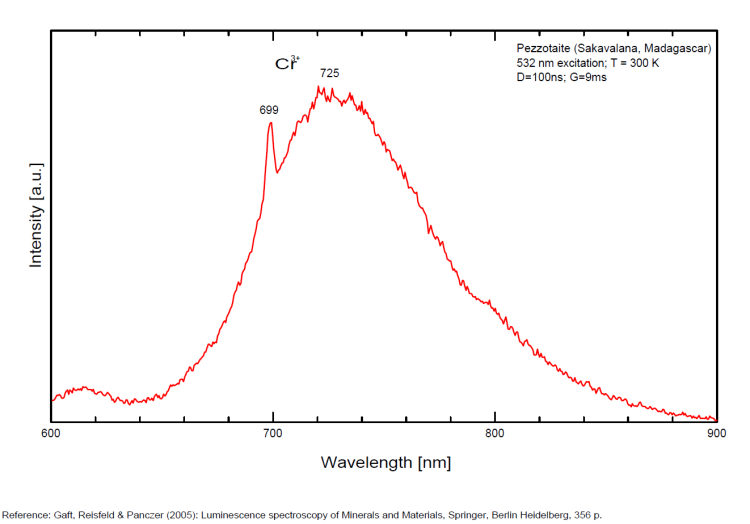
PEZZOTTAITECsBe2LiAl2Si6O18
Activator(s):
Cr3+,
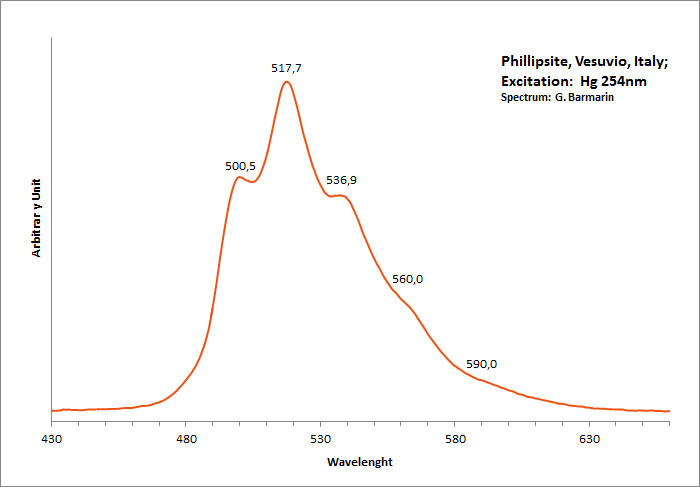
PHILLIPSITE-Ca(Ca0,5,K,Na,Ba0,5)4-7[Al4-7Si12-9O32] 12H2O
Activator(s):
(UO2)2+ (ion Uranyle) en impureté,
Peaks in the spectrum (nm):
(UO2)2+ : 467, 500, 517, 537, 560, 590, 660nm
Comments on spectra and activators:
Typical spectrum of green luminescence due to uranium impurities;
PHOSPHURANYLITE(H3O)3KCa(UO2)7O4(PO4)4 8H2O
Activator(s):
(UO2)2+ (ion Uranyle) intrinsèque ,
Peaks in the spectrum (nm):
Broad band max at 540nm (UO2)2+ : 500, 522, 546, 570, 601nm
Comments on spectra and activators:
Gorobets: broad band at room temperature max at 540 nm, 100x brighter in N liquid;
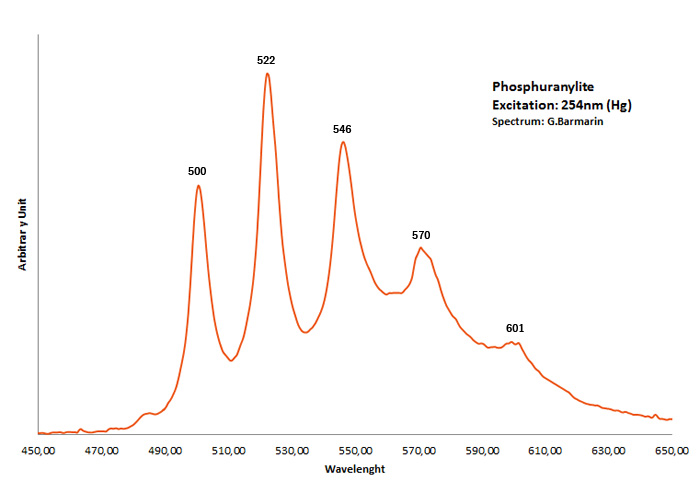
PHURCALITECa2(UO2)3O2(PO4)2 7H2O
Activator(s):
(UO2)2+ (ion Uranyle) intrinsèque ,
Peaks in the spectrum (nm):
(UO2)2+ : 521, 541, 560, 584nm
plagioclase/ Anglo-Saxon name for ALBITE/ANORTHITE serie
Activator(s):
Fe3+,
Other activators:
Pb2+, Cr3+, Eu2+, Ce3+, Sm3+, Eu3+, Tb3+, Mn2+ , Tl+,
Peaks in the spectrum (nm):
Tl+ repl. Na+ : 287nm : 321-325 347 (Ce3+),
Eu2+ : 405-412nm
Mn2+ repl.Ca2+ : broad bands peaking at 555-570nm
Fe3+ repl. [Al3+]IV : broad band at 700-750-780nm
Sm3+ : 560, 600, 645, 805nm
Eu3+ : 600-615nm
Tb3+ : 580, 620nm
Cr3+ repl. [Al3+]IV (?): 680, 683nm
Pb+ repl. Na+ : 850nm
Comments on spectra and activators:
Activators: Tl+, Pb+, Pb2+, REE3+ (Ce, Dy, Sm, Tb, Nd), Eu2+, Mn2+ (delay time: 10-12ms), Fe3+, Cr3+ (Tarashchan in Gaft)
POLYLITHIONITEKLi2AlSi4O10F2
Activator(s):
,
Peaks in the spectrum (nm):
526nm, 556,5nm, 598nm
The specified directory does not exist.
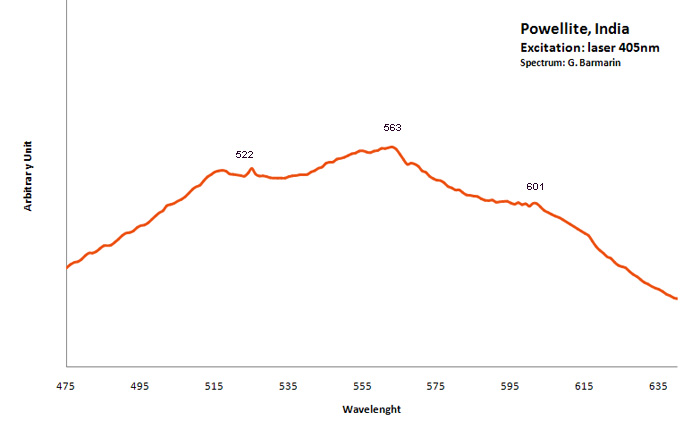
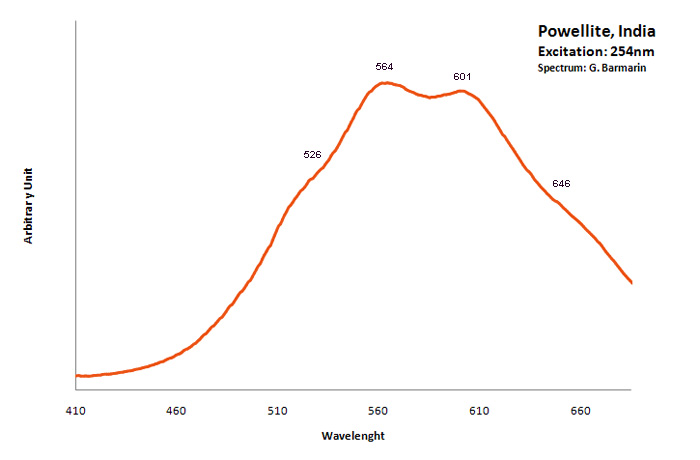
POWELLITECaMoO4
Activator(s):
MoO42-,
Other activators:
(UO2)2+ (ion Uranyle) en impureté, Er3+, Pr3+, Nd3+,
Peaks in the spectrum (nm):
MoO42- : Broad band peaking at 530 - 550 nm (Gorobets) (Lifetime: 30 μs) UO22+ : 650nm (uranyl complexes substituting to molybdenum (Panczer et al.)) Nd3+ : 810, 870nm
Comments on spectra and activators:
The natural photoluminescence emission of ordered CaMoO4 is attributed to an intrinsic slight distortion of the [MoO4] tetrahedral goup. The decay time is 200 μs . Under 532 nm cw excitation the luminescence of Pr3+, Er3+, Nd3+ as well as [UO2]2+ were revealed in powellite found associated with pitchblende and molybdenite in South Kazakhstan (Bota-Burum Mo-U deposits). (Gaft)
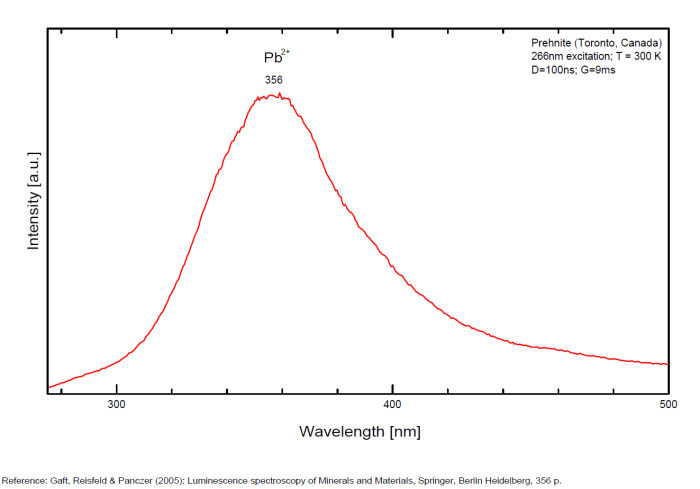
PREHNITECa2Al2Si3O10(OH)2
Activator(s):
Pb2+,
Peaks in the spectrum (nm):
Pb2+ : 356nm (exc. 266nm, t = 300ns)
Comments on spectra and activators:
Jeffrey Mine: bluish fluorescence under long wave UV illumination, plus orange-fluorescing coating by an unknown mineral (see picture above)
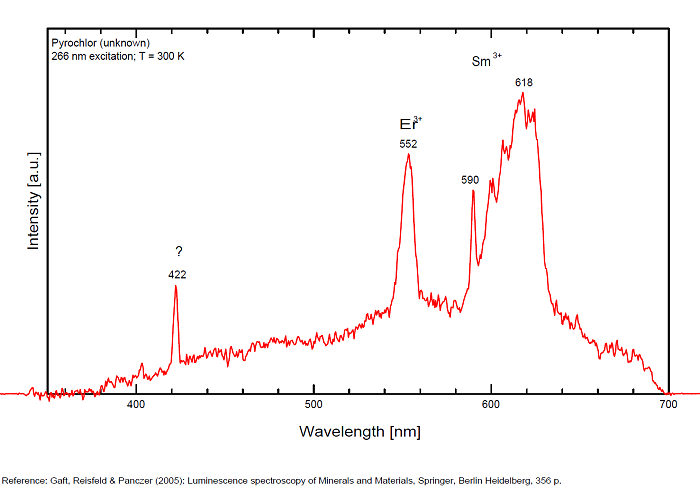
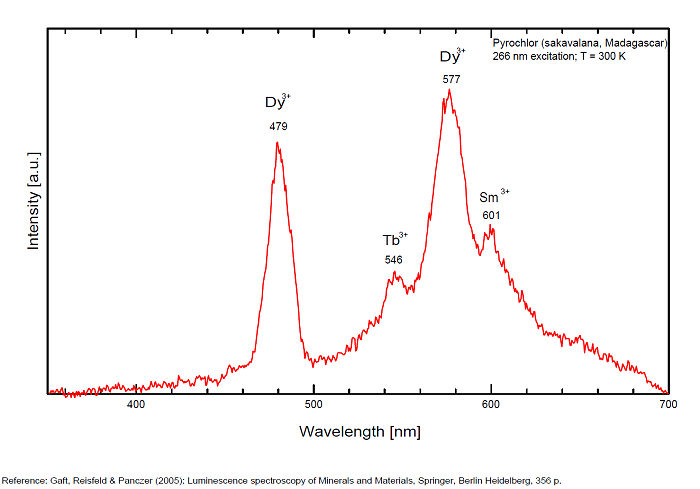
PYROCHLORE(Na,Ca)2Nb2O6(OH,F)
Activator(s):
(UO2)2+ (ion Uranyle) en impureté,
Other activators:
Sm3+, Eu3+, Dy3+, Tb3+, Nd3+,
Peaks in the spectrum (nm):
Dy3+ : 479, 577nm Tb3+ : 546nm Sm3+ : 601nm Nd3+ : 806, 867, 876, 880 - 881, 891, 1060nm
Comments on spectra and activators:
Steady-state luminescence spectra of pyrochlore revealed emission of REE, such as trivalent Dy and Nd (Gorobets and Rogojine 2001).
PYROMORPHITEPb5(PO4)3Cl
Activator(s):
Eu3+,
Other activators:
Pb2+, Ce3+, Sm3+, Eu3+, Tb3+, O-2, VO43-,
Peaks in the spectrum (nm):
(VO4)3- : Broad band peaking at 580nm Ce3+ : 350, 375nm Eu3+ : 580, 594, 611, 613, 615, 620, 622, 700nm Sm3+ : 566, 603, 652nm Nd3+ : 858, 889nm
Comments on spectra and activators:
Luminescence centers O2- (Tarashchan 1978) and evidently (VO4)3- (Gaft 1984) characterize steady-state spectra of pyromorphite. Laser-induced time-resolved technique enables to detect Ce3+, Eu3+, Sm3+ and Tb3+ emission centers and possibly Pb2+. Excitation by CW laser with 532 and 780 nm revealed narrow luminescence lines possibly belonging to trivalent REE (Nd, Eu, Sm) and two luminescence bands peaking at 625 and 675 nm (sample from Les Farges, France, unknown activator).
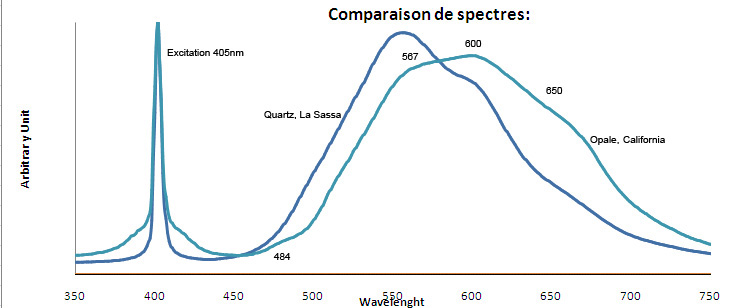
QUARTZSiO2
Activator(s):
(UO2)2+ (ion Uranyle) en impureté,
Other activators:
ST (Singlet-triplet)-Matière organique en impureté, Fe3+,
Peaks in the spectrum (nm):
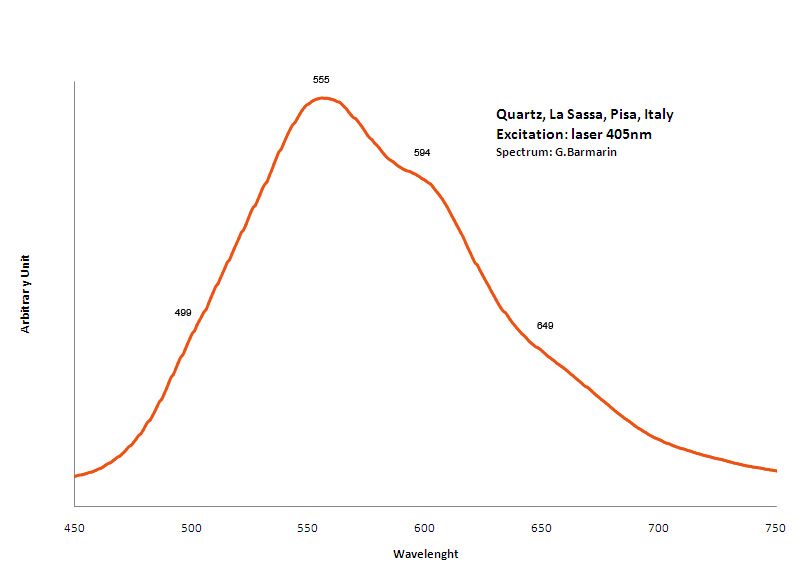
Fe3+ : 680 nm (Gorobets) Oil inclusions : 437, 489, 512, 546, 601nm Quartz from La Sassa, Italy: 500, 520, 555, 595, 666nm
Comments on spectra and activators:
Cathodoluminescence: blue or yellow.
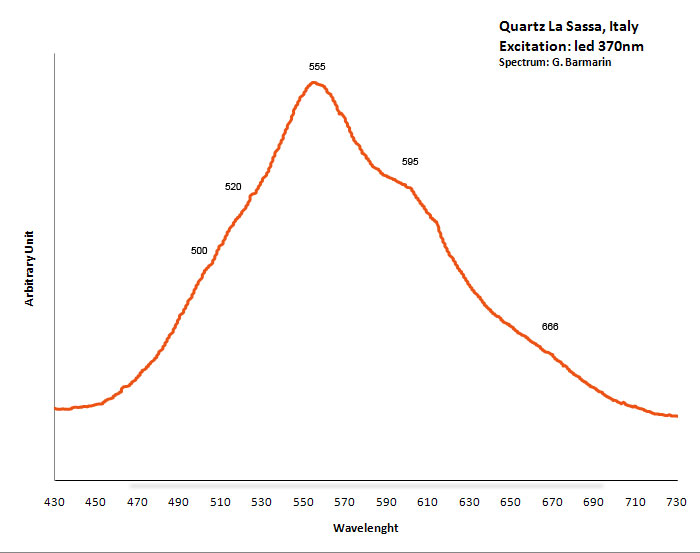
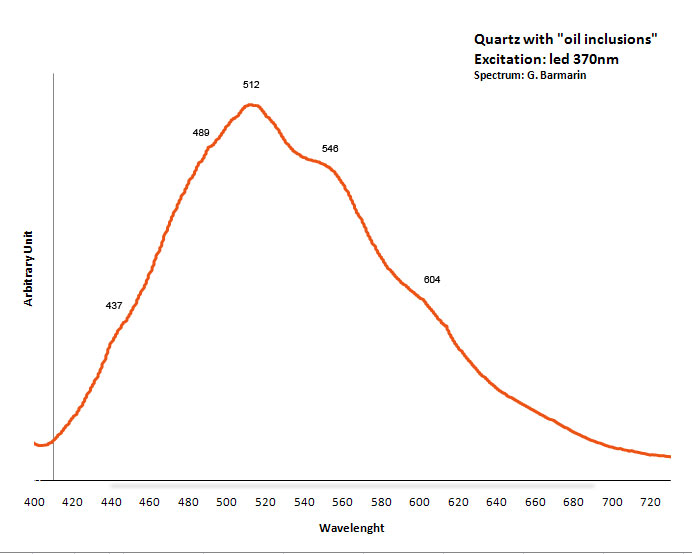
Excitation: led 370nm. Col. G. Barmarin; Spectre: G. Barmarin
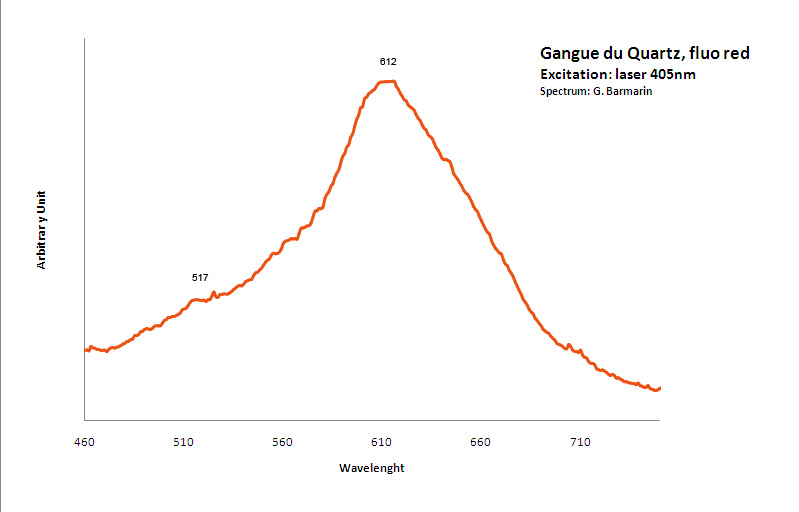
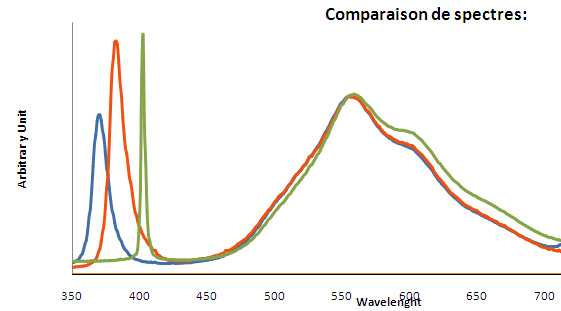
RAVATITEC14H10
Activator(s):
Matière organique intrinsèque,
Peaks in the spectrum (nm):
395, 408, 433, 459, 486nm
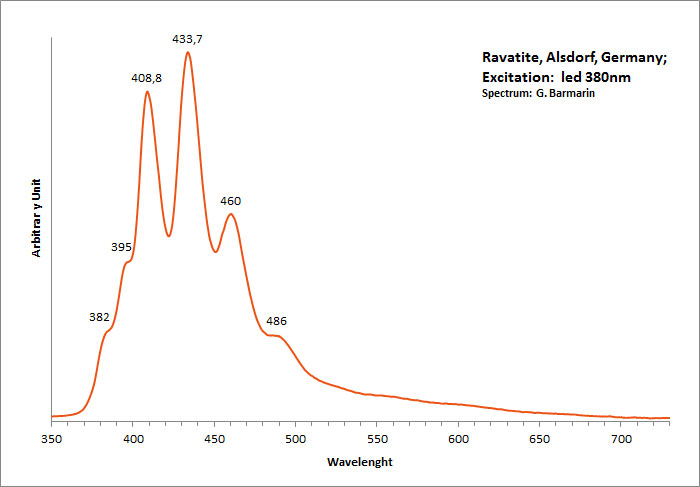
Excitation: led 380nm. Col. G. Barmarin; Spectre: G. Barmarin
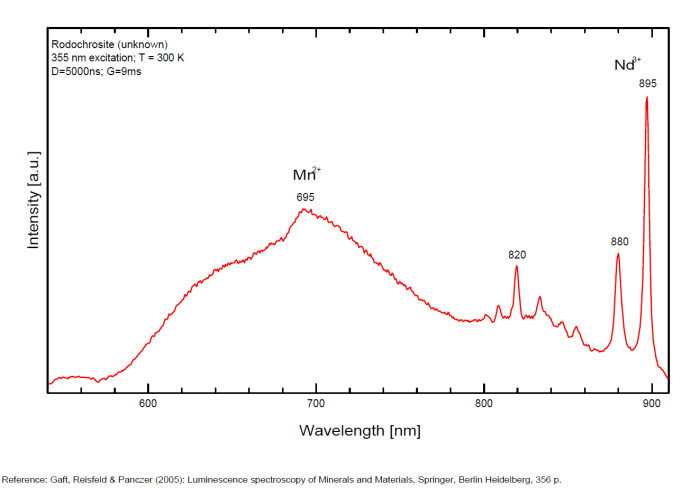
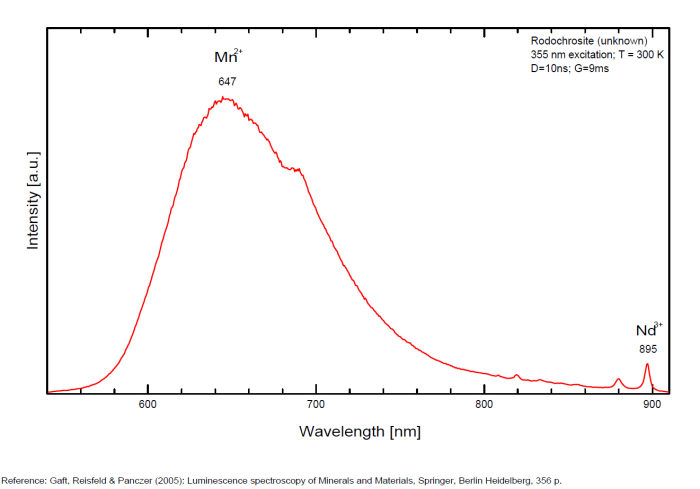
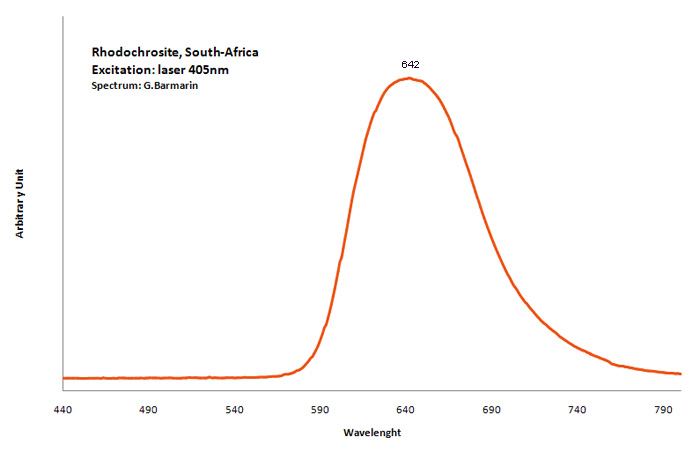
RHODOCHROSITEMn+2CO3
Activator(s):
Mn2+ ,
Other activators:
Nd3+,
Peaks in the spectrum (nm):
Mn2+ : Large band peaking at 642-647nm (100nm half-width) Mn2+ : large band peaking at 695nm Nd3+ : 820, 880, 895nm
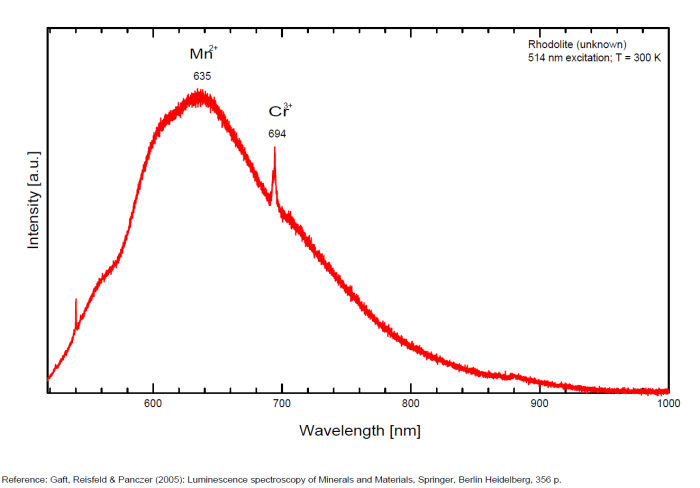
rhodolite
Activator(s):
Mn2+ ,
Other activators:
Cr3+,
Peaks in the spectrum (nm):
Mn2+ sustituting to Ca2+ : Broad band peaking at 635 nm Cr3+ replacing Al3+ : line at 694 nm, band at 720nm , 690, 703, 733nm
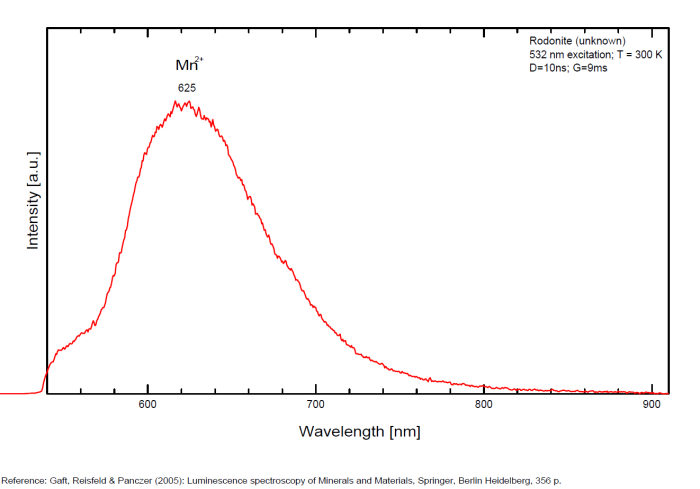
RHODONITE(Mn+2,Fe+2,Mg,Ca)SiO3
Activator(s):
Mn2+ ,
Peaks in the spectrum (nm):
Mn2+ : band peaking at 625nm (100nm half-width)
Ruby
Activator(s):
Cr3+,
Peaks in the spectrum (nm):
Cr3+ replacing Al3+ : Lines at 692.8 (693), 694.3nm (sharp R1 and R2 lines) Cr3+ : 658.2 , 668.2 , 674.1 , 680 (small lines) N-lines (Cr3+ pairs) : 705.8 , 712nm (small lines)
Comments on spectra and activators:
Activator: Cr3+ replacing Al3+ giving strong well known 2E->4A2 lines with long decay time; Besides that, much weaker narrow lines present, which are connected with Cr-pairs and more complicated complexes (so called N-lines) (Tarashchan 1978). Lifetime of the R-line: 3,6ms;
The specified directory does not exist.
SABUGALITEH0,5Al0,5(UO2)2(PO4)2 8H2O
Activator(s):
(UO2)2+ (ion Uranyle) intrinsèque ,
Peaks in the spectrum (nm):
(UO2)2+ : 491.4, 506.4, 527.6, 550.7, 576.1, 604.8 / (484nm), 503nm, 523nm, 547nm, 572nm, 601nm, 631nm (?)
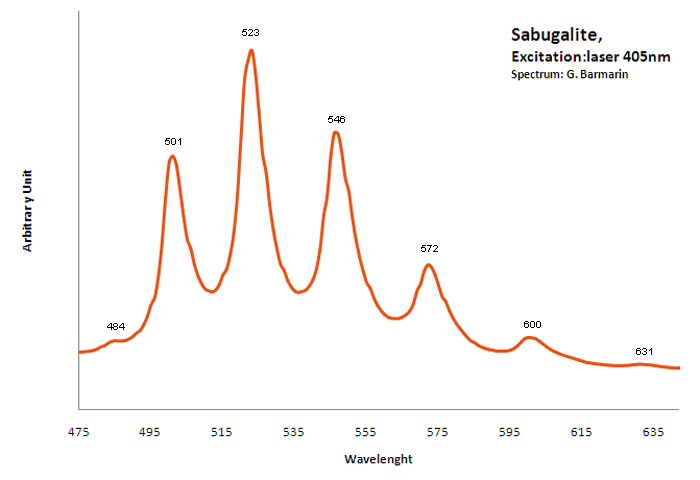
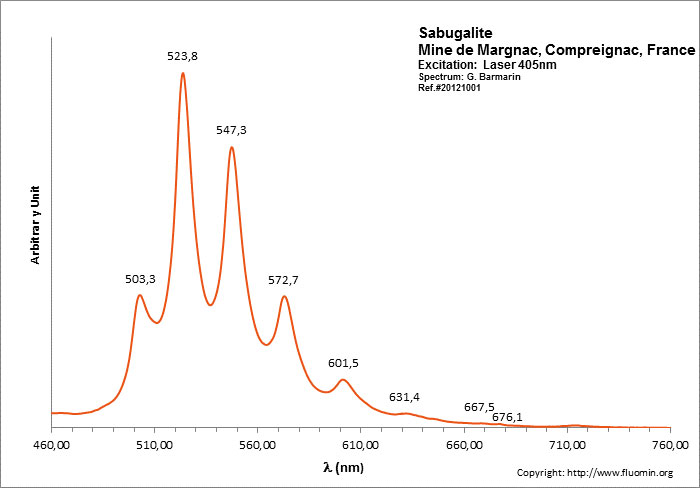
SALEEITEMg(UO2)2(PO4)2 10H2O
Activator(s):
(UO2)2+ (ion Uranyle) intrinsèque ,
Peaks in the spectrum (nm):
[ 489.0 , 501.1 , 522.1 , 545.7 , 570.9 , 600.9 (Geipel) ] [498nm, 519nm, 542nm, 567nm, 595nm (Sample in collection)]
Excitation: UVSW 254nm. Col. G. Barmarin; Spectre: G. Barmarin
scapolite/ (Group: MEIONITE, MARIALITE )
Activator(s):
Fe3+,
Other activators:
Pb2+, S2-, Eu2+, Mn2+ , O-2,
Peaks in the spectrum (nm):
Fe3+? : band at 710 - 720nm Mn2+ : broad band at 630 - 690nm
Comments on spectra and activators:
Red fluorescence: Fe3+? (band at 710 - 720nm) Mn2+ (broad band at 630 - 690nm) Blue fluorescence: Pb2+ (Langban, Sweden) A recent find of colorless scapolite in Badakhshan ( Afghanistan) turns to a blue color when exposed to UVSW.
SCHEELITECaWO4
Activator(s):
WO42-,
Other activators:
Sm3+, Eu3+, Dy3+, Ho3+, Er3+, Tb3+, Pr3+, Nd3+, Yb3+, Tm3+,
Peaks in the spectrum (nm):
WO42-: Broad band centered at 425 - 435nm (Lifetime: 9μs @ 405nm)
Tb3+: 439nm
Dy3+: 488, 575nm
Sm3+: 609, 647nm
Pr3+: 607nm
Comments on spectra and activators:
Scheelite is characterized by broad luminescent bands centered at 425 - 435 nm (blue emission) of intrinsic activator (WO4)2- groups and impurity (MoO4)2- groups. Those broad bands are attributed to an intrinsic slight distortion of the [WO4]/ [MoO4] tetrahedral group. [WO4] lifetime: 9μs (@ 405nm). Such strong bands prevent in many cases the detection of lines of rare-earth elements, especially Tm, Er and Ho, which have weak luminescence in the corresponding spectral range. Yet, scheelite incorporates often tens to thousands of ppm RRE in substitution for Ca giving sometimes typical peaks in the fluorescence spectrum. Visible peaks in relation with the presence of REE: 488 et 575 (Dy3+), 609 et 647 (Sm3+), 439 (Tb3+) et 607 (Pr3+). But the lines of certain REE may be hidden by stronger luminescence of others REE. For example, the luminescence of Pr3+ is difficult to detect because its radiative transitions are hidden by the lines of Sm3+, Dy3+ and Nd3+, the luminescence of Tm3+ is concealed by Tb3+ and so on. The pegmatitic and hydrothermel scheelite shows the lines of Erbium and Terbium, while scheelite occurrences related to eruptive complex and sulfide ore shows dominantly the lines of the REE of the Cerium group. Under cw laser excitation at 532 several narrow lines have been found in red and IR part of the spectrum connected with Nd3+ and possibly Sm3+ centers. Green emission due to (MoO4)2-(Tarashchan) or possibly Pb (Blasse). The color of the fluorescence of scheelite gives an idea of the unwilled presence of molybdenium in the ore. Concentrate of scheelite not penalized for molybdenium have a distinct blue fluorescence color. Those that fluoresce white are borderline and contain roughly 0,35% to 1% of Mo. And scheelite that fluoresces distinctly yellow contains more than 1% corresponding to a transition to powellite. Higher than 4,8%, the yellow fluorescence color stays unchanged and cannot anymore be used as an indication of the percentage of Mo. Using this property, a method was developped by R.S. Canon jr. (1942) while studying tungsten deposits in the seven Devils mining district of Idaho. A serie of finely powdered synthetic preparation or natural ore of known composition are permanently mounted in circular areas on a black card, being placed in order of increasing molybdenium content. There are twelve standard values on the card: 0,05, 0,19, 0,33, 0,48, 0,72, 0,96, 1,4, 2,4, 3,4, and 4,8% plus a pure calcium molybdate (48% Mo). Alternating with the covered circle are circular holes of the same size. The card is used by placing a hole over a powdered sample of the scheelite ore to be tested and comparing the fluorescence color of the sample with those of the adjacent standards. The sample will be found to have a fluorescence color according or between two standards and hence the approximate composition could be defined.
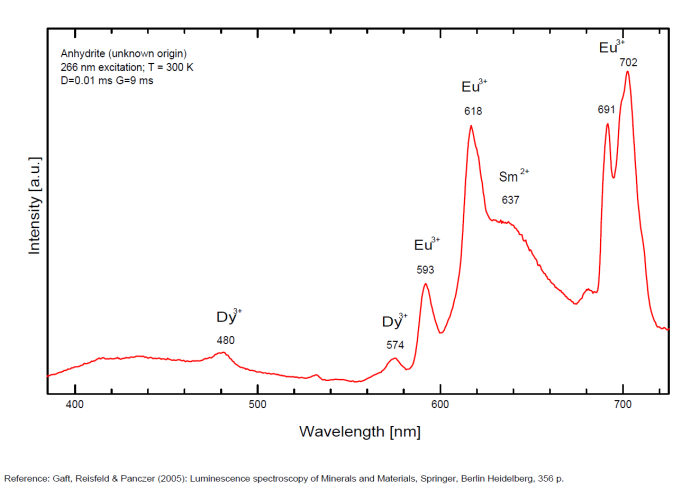
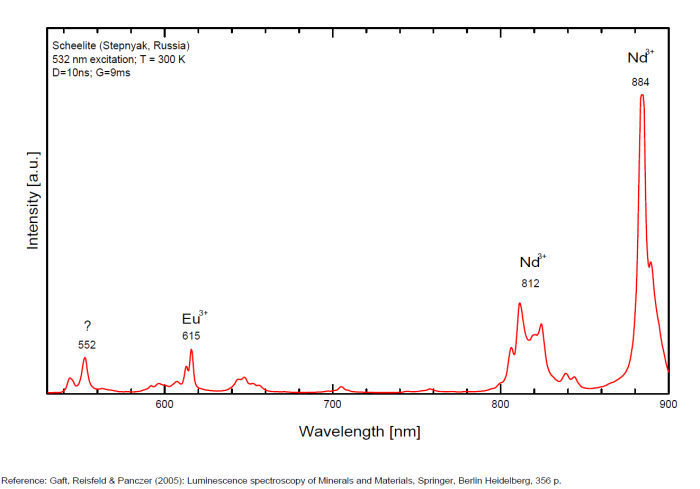
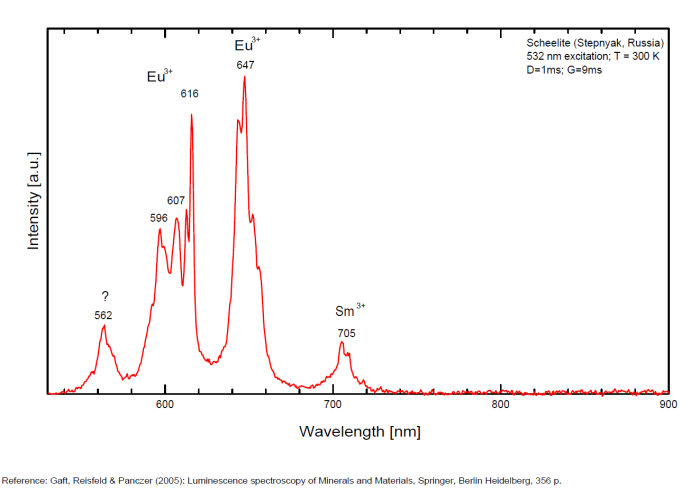
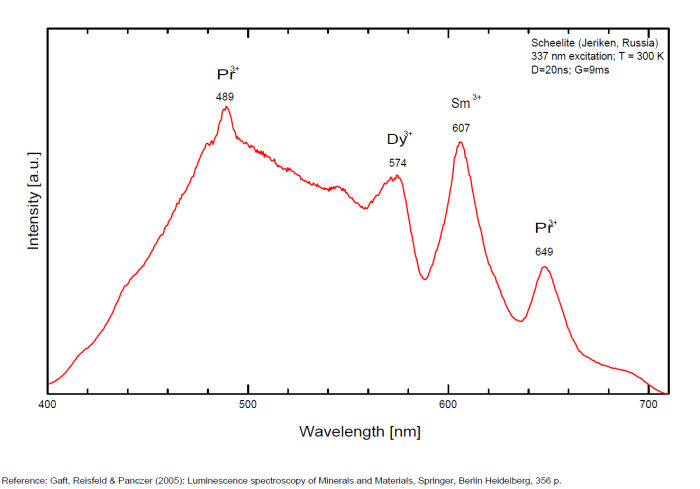
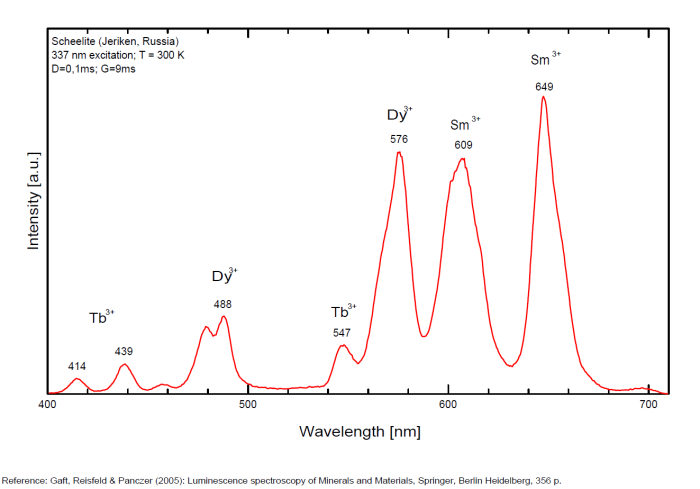
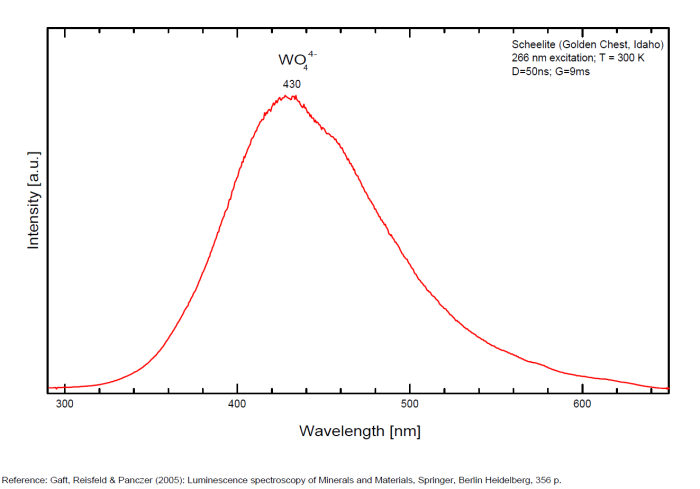
SCHROECKINGERITENaCa3(UO2)(SO4)(CO3)3F 10H2O
Activator(s):
(UO2)2+ (ion Uranyle) intrinsèque ,
Peaks in the spectrum (nm):
465nm, 483nm, 504nm, 526nm, 550nm, 577nm, 608nm, 641nm, 676nm, 713nm
Comments on spectra and activators:
Lifetime: 125μs @505nm;
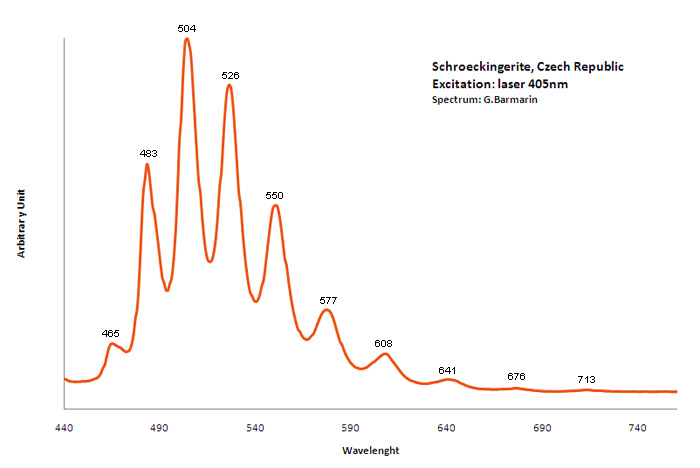
SKLODOWSKITE(H3O)2Mg(UO2)2(SiO4)2 4H2O
Activator(s):
(UO2)2+ (ion Uranyle) intrinsèque ,
Peaks in the spectrum (nm):
(UO2)2+ : (503nm), 522nm, 546nm, (597nm)
Comments on spectra and activators:
Nature of the sample to be verified.
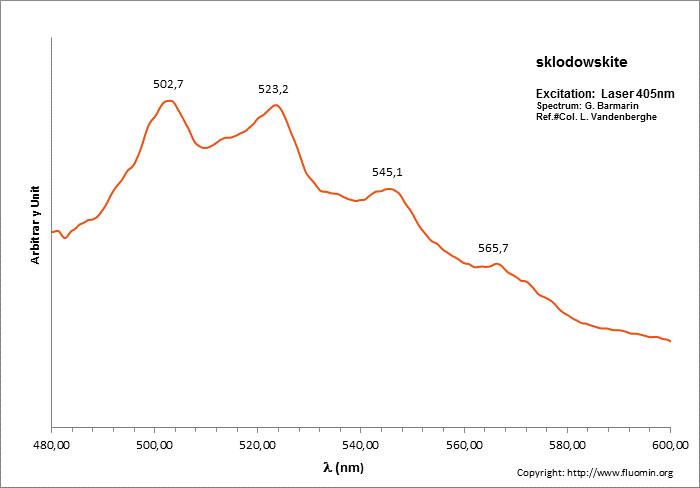
SODALITENa8Al6Si6O24Cl2
Activator(s):
S2-,
Other activators:
(UO2)2+ (ion Uranyle) en impureté, Fe3+, Mn2+ ,
Peaks in the spectrum (nm):
S2- : 587, 608, 628, 653, 677, 707, 732nm Fe3+? repl. Al3+ : 687-720nm (UO2)2+ : 495, 515, 537nm Mn2+ repl. Na+ : 650nm
Comments on spectra and activators:
Some time Green Luminescence due to uranium impurities; Cathodoluminescence: intensive greenish-blue or yellow-green or orange. The research history of the yellow-orange luminescence of sodalite was well presented by Sidike et al (2007). It may be summarized that published data on the yellow-orange luminescence spectra of sodalite was ascribed to S2- which was confirmed by the study of synthetic analogs activated by sulfur. Nevertheless, Sidike et al. (2007) noted that the peak wavelengths in the yellow orange band reported by many investigators for sodalite and ascribed to S2-do not agree. It was never reported by single author on several types of S2- luminescence spectra from the same deposit, which may be explained by different chemical composition and impurities, but different spectra were ascribed to the S2- luminescence center in sodalite samples from the same deposit. The values for luminescence maxima were reported to range from 630 nm to 708 nm. A similar situation takes place in scapolite, where the peak wavelengths of S2- emission bands reported by different authors do not agree (Sidike et al. 2008). It was proposed that the main reason for these discrepancies is probably the unsuitable correction of the measured spectra. Nevertheless, it is difficult to assume that such strong discrepancies may be explained only by the absence of proper calibration. A possible explanation for the differences between published spectra may be the presence of several different S2- centers, or the presence of the luminescence centers with vibrational structure of other types. (Gaft) The crystal structure of sodalite is an ordered framework of linked AlO4 and SiO4 tetrahedra in which Si and Al alternate on the tetrahedral sites. The overall linkage of (Al,Si)O4 tetrahedra results in cubo-octahedral cavities, which contain a centrally placed anion coordinated tetrahedrally to four cations. The flexibility of the sodalite structure allows a wide range of cations and anions with potential luminescence ability to be substituted into it. Thus, many impurities with potential luminescence ability besides S2- may be present in the sodalite structure, such as Fe3+ in Al and Si positions, Mn2+ in Na or Be positions, Pb2+ or Tl+ in the Na position and so on. (Gaft) By Laser-induced time-resolved technique Gaft was able to detect two Fe3+ or Cr3+ (broad band at 687 - 700 & 725 nm), possibly Pb2+ (broad band at 440nm), Eu2+ (band at 405nm), Ce3+ ( band at 340nm) and S2- (broad band at 600 - 620 - 653 - 655nm with weak vibrational structure) emission centers (Gaft) The photoluminescence and excitation spectra of sodalites from Greenland, Canada and Xinjiang (China) are observed at 300 and 10 K in detail. The features of the emission and excitation spectra of the orange-yellow fluorescence of these sodalites are independent of the locality. The emission spectra at 300 and 10 K consist of a broad band with a series of peaks and a maximum peak at 648 and 645.9 nm, respectively. The excitation spectra obtained by monitoring the orange-yellow fluorescence at 300 and 10 K consist of a main band with a peak at 392 nm. The luminescence efficiency of the heat-treated sodalite from Xinjiang is about seven times as high as that of untreated natural sodalite. The emission spectrum of the S2 − center in sodalite at 10 K consists of a band with a clearly resolved structure with a series of maxima spaced about 560 cm−1 (20–25 nm) apart. Each narrow band at 10 K shows a fine structure consisting of a small peak due to the stretching vibration of the isotopic species of 32S34S−, a main peak due to that of the isotopic species of 32S2 − and five peaks due to phonon sidebands of the main peak. (see Aierken Sidike, Alifu Sawuti, Xiang-Ming Wang, Heng-Jiang Zhu, S. Kobayashi, I. Kusachi, N. Yamashita, Fine structure in photoluminescence spectrum of S2− center in sodalite, Physics and Chemistry of Minerals, September 2007, Volume 34, Issue 7, pp 477–484 ) The emission and excitation spectra of yellow luminescence due to S2− in scapolites (from Canada and from an unknown locality) were observed at 300, 80 and 10 K. Emission and excitation bands at 10 K showed vibronic structures with a series of maxima spaced 15–30 and 5–9 nm, respectively. The relative efficiency of yellow luminescence from scapolite #2 was increased up to 117 times by heat treatment at 1,000°C for 2 h in air. The enhancement of yellow luminescence by heat treatment was ascribed to the alteration of SO32− and SO4 2− to S2 − in scapolite. (see Aierken Sidike, I. Kusachi, S. Kobayashi, K. Atobe, N. Yamashita, Photoluminescence spectra of S2− center in natural and heat-treated scapolites, Physics and Chemistry of Minerals, April 2008, Volume 35, Issue 3, pp 137–145 )
SODDYITE(UO2)2SiO4 2H2O
Activator(s):
(UO2)2+ (ion Uranyle) intrinsèque ,
Peaks in the spectrum (nm):
(UO2)2+ : 558, 572nm
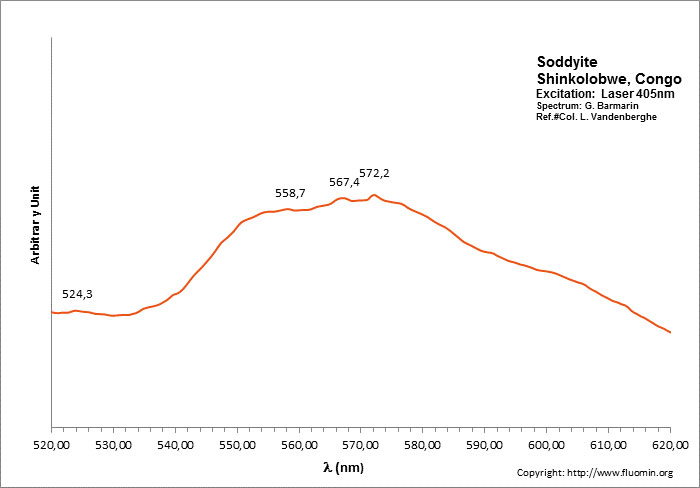
SODIUM URANOSPINITE(Na2,Ca)(UO2)2(AsO4)2 5H2O
Activator(s):
(UO2)2+ (ion Uranyle) intrinsèque ,
Peaks in the spectrum (nm):
502nm, 524nm, 548nm, 573nm, 601nm
SODIUM-ZIPPEITENa4(UO2)6(SO4)3(OH)10 4H2O
Activator(s):
(UO2)2+ (ion Uranyle) intrinsèque ,
Peaks in the spectrum (nm):
523nm, 537nm
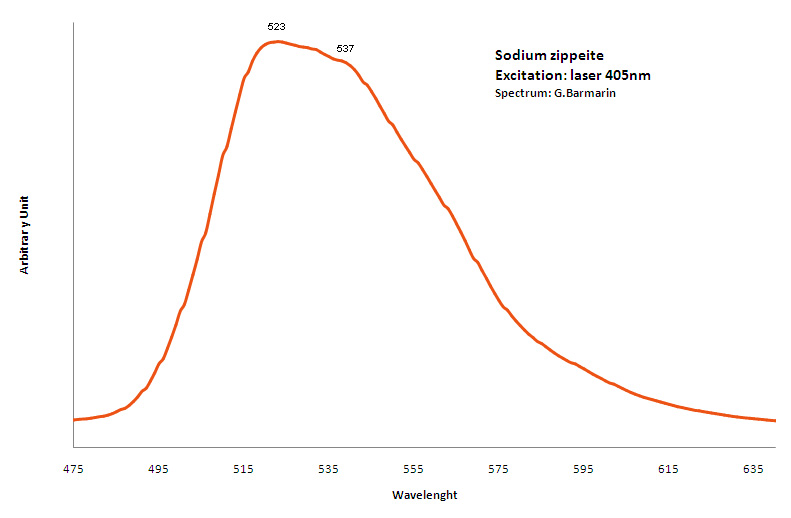
SPESSARTINEMn3+2Al2(SiO4)3
Activator(s):
Cr3+,
Other activators:
Mn4+,
Peaks in the spectrum (nm):
Cr3+ : lines at 606, 627, 694, 707nm Mn4+ ? : band around 650nm
Comments on spectra and activators:
Laser excitation;
SPINELLEMgAl2O4
Activator(s):
Cr3+,
Other activators:
Mn2+ ,
Peaks in the spectrum (nm):
Cr3+ : lines at 676 (675), 686 (685), 698 (697), 708 (707), 712, 718 (717) nm
Mn2+ : broad band peaking at 550-612 nm
Comments on spectra and activators:
Activator: Cr3+ and possibly Mn2+ (broad band peaking at 612 nm or emissions bands with max from 550 to 620nm). The fluorescence of spinel was studied as early as 1887 by De Boisbaudran who found a red glow in most specimens and some green fluorescence in a few. Crookes plotted in 1887 the red fluorescence spectra of spinel and pointed out the particular sharp band at 685,7nm. Nichols studied the cathodoluminescence of spinel in 1928.
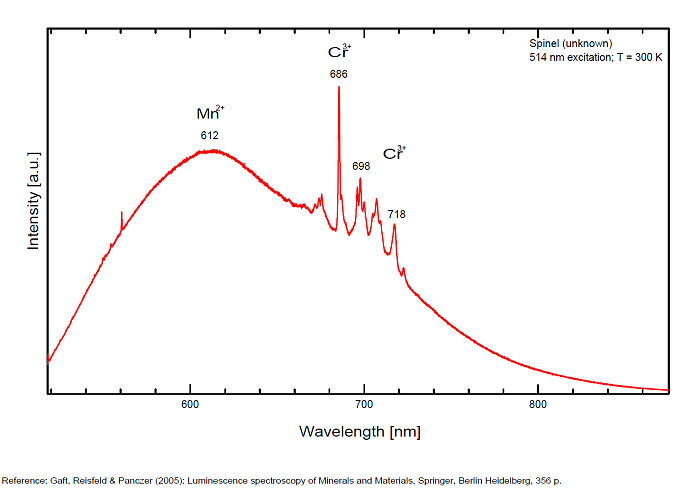
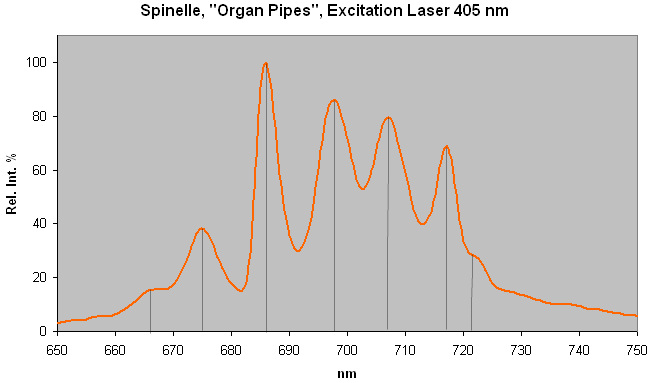
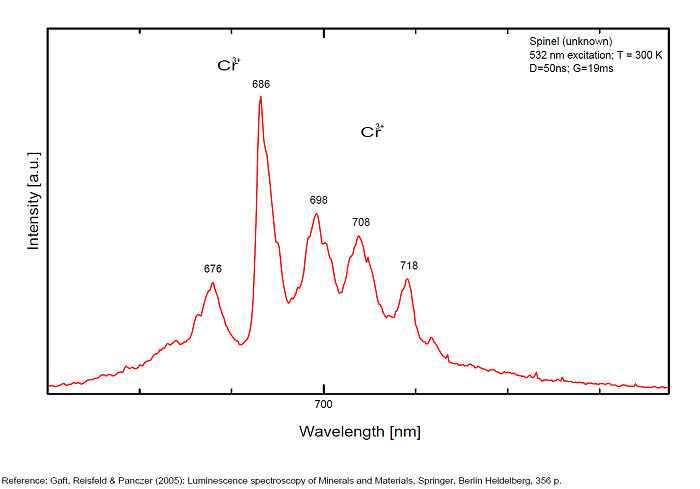
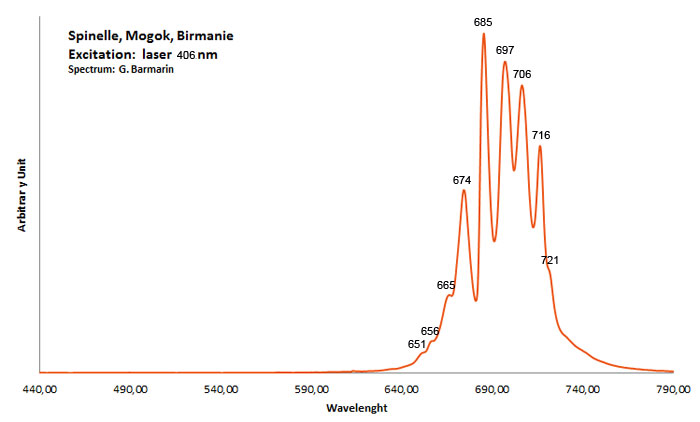
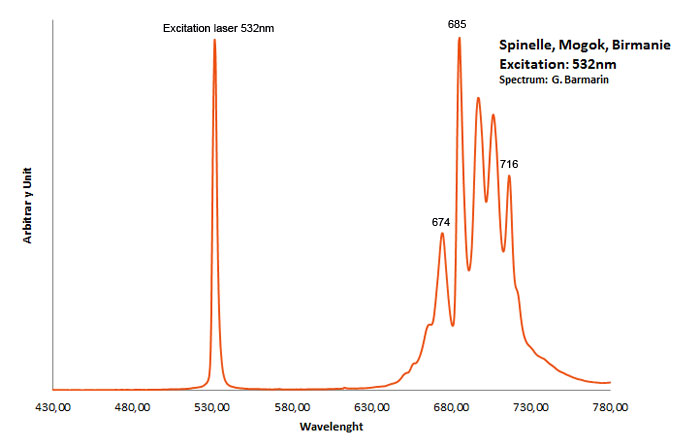
SPODUMENELiAlSi2O6
Activator(s):
Mn2+ ,
Other activators:
Cr3+, Fe3+,
Peaks in the spectrum (nm):
Mn2+ substituting for Li : broad band around 595 - 615nm (10ms) Cr3+ : 676, 690nm (peak) Fe3+ : 685nm (5 ms)
Comments on spectra and activators:
Mn2+ is shown to be mainly in Li-sites rather than Al-sites and gives rise to a broad emission centered at 600 nm. In Cr-rich crystals only one R1 line is observed and Cr3+ emission is evident at room temperature. In green hiddenite crystals, Cr3+ emission is dominant at room temperature where the R-lines are superimposed on a broad-band emission. (Source: Luminescence spectroscopy of Cr3+ and Mn2+ in spodumene (LiAlSi2O6), WALKER G. see below). Spodumen is a monoclinic pyroxene with two not equivalent metal cation sites M1 and M2. The aluminum occupies the smaller M1 site, which is approximately octahedral (actual symmetry C2) with an average metal-oxygen distance of 1.92 A. The M2 site, occupied by Li, is also six-fold coordinated with an average metal-oxygen distance of 2.23 Å. Both Al and Li sites may be substitutionally replaced by ions of the transitional metals in various proportions. Both Mn2+ and Cr3+ centers have been identified in luminescence spectra by steadystate spectroscopy (Tarashchan 1978; Walker et al. 1997). Besides, long lived deep red luminescence was found supposedly generated by Fe3+ especially in the specimen fluorescing a bright lilac color under SW, light peach LW and having a violet (not pink) color in daylight. At room and lower temperatures only one emission band of Mn2+ occurs. It is very probable that Mn2+ ions in the spodumen matrix is present only in one site and that Mn2+ is mainly in Li-sites rather than Al-sites. (Gaft)
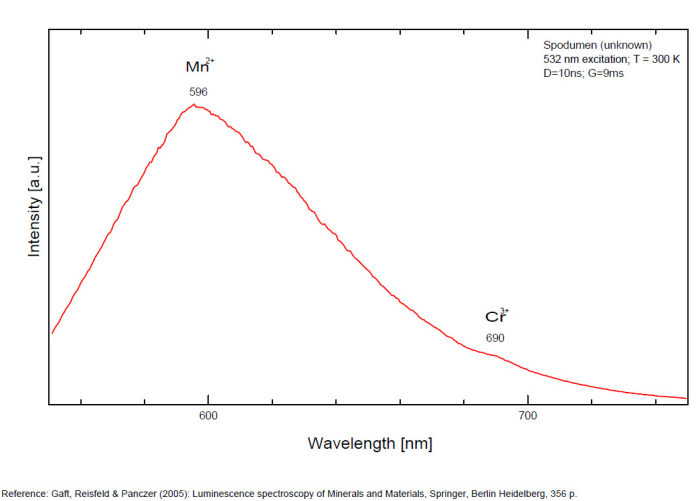
SULFOHALITENa6(SO4)2FCl
Activator(s):
,
Peaks in the spectrum (nm):
490nm, 516nm, 548nm, 598nm,654nm

SWARTZITECaMg(UO2)(CO3)3 12H2O
Activator(s):
(UO2)2+ (ion Uranyle) intrinsèque ,
Peaks in the spectrum (nm):
472.3, 488.9, 509.0, 531.1, 554.7, 578.9nm

TALMESSITECa2Mg(AsO4)2 2H2O
Activator(s):
(UO2)2+ (ion Uranyle) en impureté,
Peaks in the spectrum (nm):
UO22+ : (490), 502, 521, 544, (566), (595nm)
Comments on spectra and activators:
Spectrum with typical peaks of Uranyl impurities;
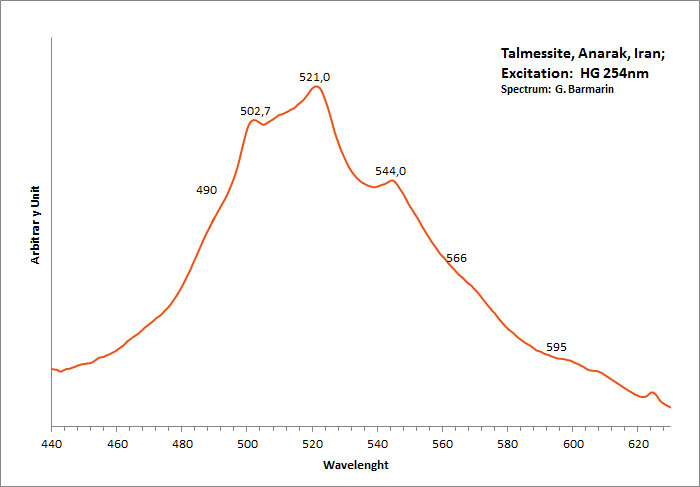
tetranatrolite
Activator(s):
S2-,
Peaks in the spectrum (nm):
S2- : 564, 590, 606, 622, 646, 667, 687, 715, 745nm
Comments on spectra and activators:
Typical spectrum of S2-;
spectre.jpg)
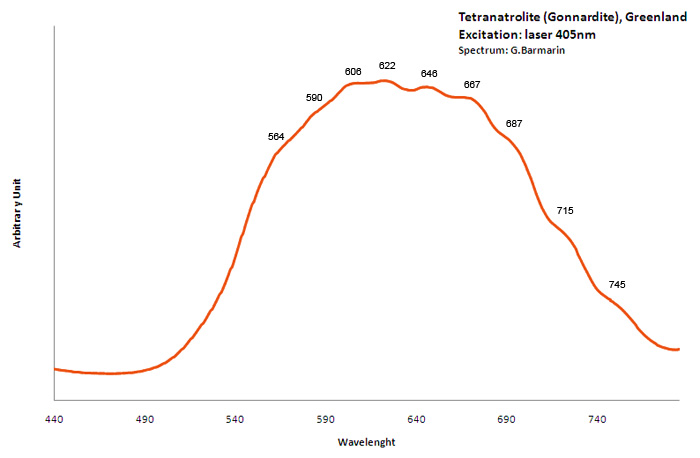
THORITE(Th,U)SiO4
Activator(s):
(UO2)2+ (ion Uranyle) en impureté,
Other activators:
Sm3+, Eu3+, Dy3+, Tb3+, Ti - O,
Peaks in the spectrum (nm):
(UO2)2+ : broad band peaking at 525 - 530nm (FWMH: 75nm) Eu3+ : lines at 591, 615 and 702nm Sm3+ : 602, 640nm (Ti - O) : large band with max at 520-550nm
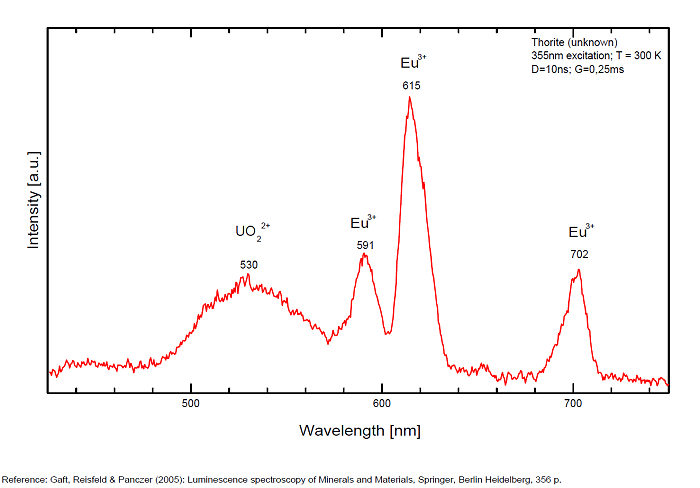
Tiffany stone
Activator(s):
(UO2)2+ (ion Uranyle) en impureté,
Peaks in the spectrum (nm):
UO22+ : 504, 524, 548, 572, 601nm
Comments on spectra and activators:
Green fluorescence due to the presence of trace of uranium in chalcedony (Typical spectrum of Uranyl)
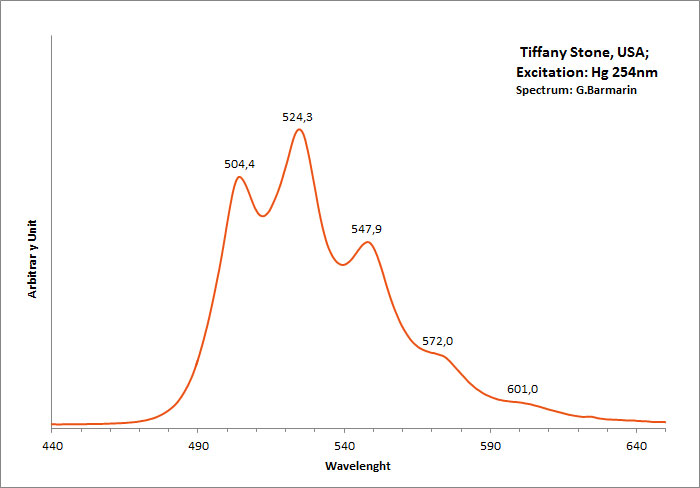
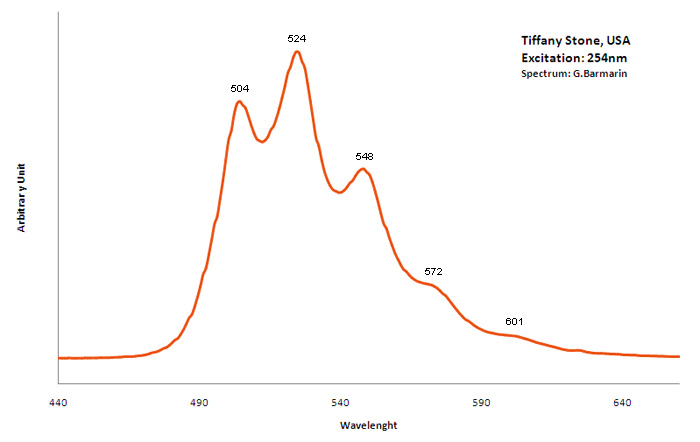
TITANITECaTiSiO5
Activator(s):
Centres dûs aux effets des radiations,
Other activators:
Cr3+, Ti3+, Sm3+, Eu3+, Er3+, Pr3+, Nd3+, Tm3+,
Peaks in the spectrum (nm):
Sm3+ : 600nm Eu 3+ : 620, 703nm Ti3+? : 742 749 762 769 793 798 804 820nm Cr3+ : 750, 750, 786nm
Comments on spectra and activators:
Potential luminescent centers include intrinsic TiO6 and Ti3+ and different impurities such as trivalent and divalent rare-earth elements (REE), Pb2+ and Mn2+ substituting for Ca2+, Cr3+ and Mn4+ substituting for Ti4+, and Cr4+, Cr5+ and Fe3+ substituting for Si4+. Besides that, titanite can incorporate minor amounts of radioactive impurity components (particularly U and Th) that affect the crystal structure through α- and β-decay events. Thus radiation-induced luminescence centers are also possible. Nevertheless, steady-state luminescent spectroscopy of titanite under UV and X-ray excitations did not reveal characteristic bands and lines (Gorobets and Rogojine 2001) and only ionoluminescence spectrum of titanite exhibits various narrow lines of Sm3+, Eu3+ and Nd3+ (Yang 1995). Chromium and other metals, such as Nb, Ta, V, Mn, Mg, Sn, Al, and Fe, are generally considered to be incorporated at the six-fold coordinated Ti-site whereas REEs substitute Ca on its large, seven-coordinated site. (Gaft)
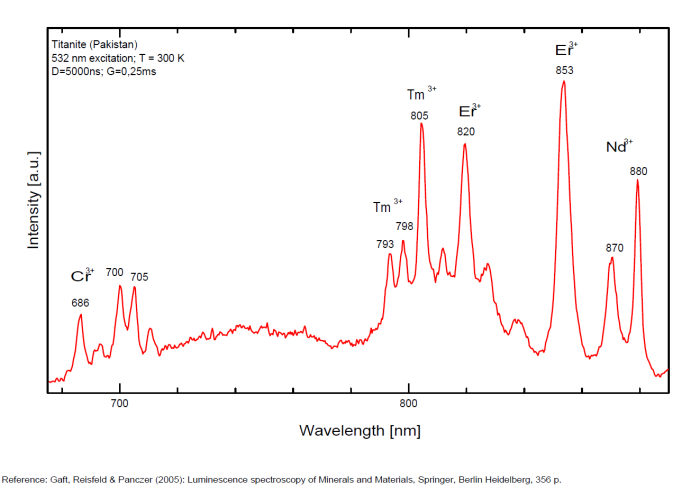
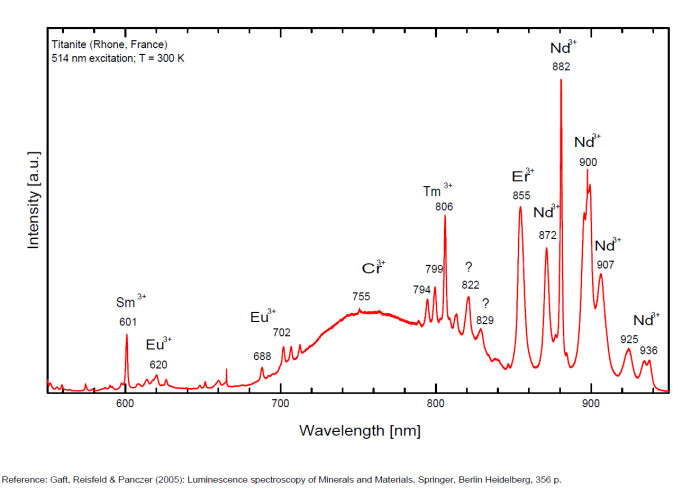
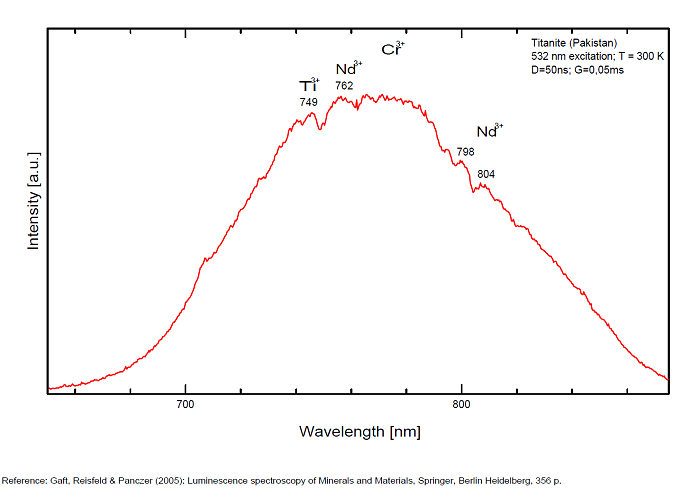
TOPAZEAl2SiO4(F,OH)2
Activator(s):
TiO6,
Other activators:
Cr3+,
Peaks in the spectrum (nm):
TiO6 : broad band peaking at 455nm Cr3+ : lines at 680, 684, 695 - 697, 711, 734nm
Comments on spectra and activators:
Broad band peaking at 455 nm (TiO6). lines at 680, 684, 695-697, 711, 734 and band peaking near 680 nm, both due to Cr3+ It is established by Tarashchan that all photoluminescence characteristics of variously colored topazes from Ouro Preto, Brazil are due to three structurally non-equivalent Cr3+ centers isomorphically substituting Al3+ in the topaz structure and forming [CrO4F2]7−, [CrO4OH,F]7−, and [CrO4(OH)2]7− complexes. (Source: Luminescence spectroscopic study of Cr3+ in Brazilian topazes from Ouro Preto by A. N. Tarashchan and Al. see below). Laser-induced time-resolved technique enables to detect three different Cr3+ and possibly Mn4+ and (TiO4) emission centers (Gaft et al. 2003a)
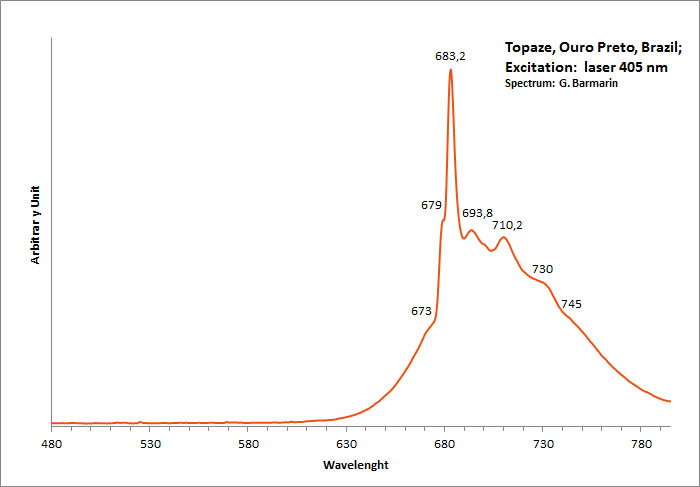
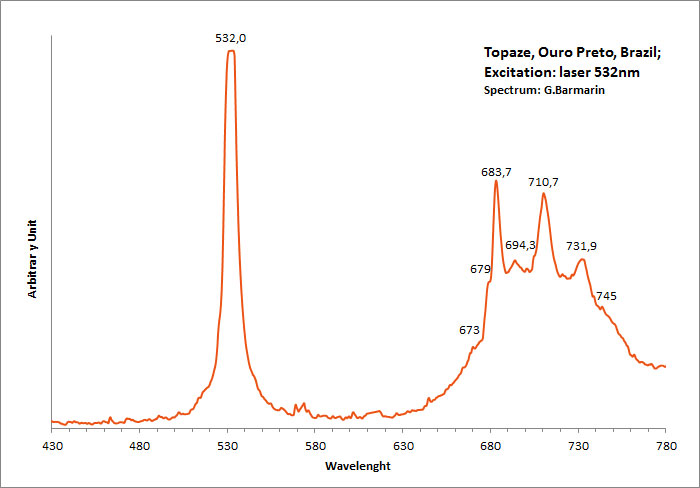
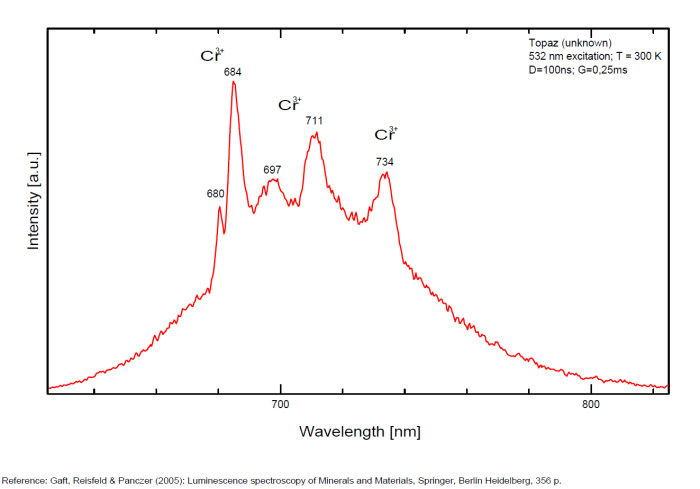
TOUNKITE(Na,Ca,K)8 (Al6 Si6 O24) (SO4)2 Cl H2 O
Activator(s):
S2-,
Comments on spectra and activators:
Typical spectrum of S2- activator.
Source: Minerals 2024, 14(4), 382; https://doi.org/10.3390/min14040382
tourmaline/ (Group: see/voir ELBAITE, UVITE, DRAVITE)
Activator(s):
Cr3+,
Other activators:
Fe3+, Mn2+ ,
Peaks in the spectrum (nm):
Cr3+ : lines at 691, 697, 707 nm
Fe3+ : band at 700 - 750 nm
Mn2+ : band at 560 - 570 nm
Comments on spectra and activators:
Under lamps excitation tourmaline is practically non-luminescent, while under X-ray excitation it exhibits impurity luminescence from Fe3+ centered at 700–750 nm and Mn2+ centered at 560–570 nm (Kusnetsov and Tarashchan 1988). (Gaft) Activators: Cr3+ (lines at 691, 697, 707 nm), Fe3+ (band at 720 - 750 nm), Mn2+ (band at 560 - 570 nm)
TUGTUPITENa4AlBeSi4O12Cl
Activator(s):
S2-,
Other activators:
Fe3+, Eu2+, Ce3+, Mn2+ ,
Peaks in the spectrum (nm):
S2- : band with weak vibrational structure peaking at 600-680nm Fe3+repl. Si4+ : 570 - 680nm
Comments on spectra and activators:
It is generally accepted that the yellow-orange emission of tugtupite with weak vibrational structure at 300 K, which becomes more prominent at low temperatures, is connected to a S2- luminescence center (Povarennykh et al. 1971). Laser-induced time-resolved technique enables Gaft to detect Eu2+ (Band at 430nm) and Ce3+ (Band at 360nm), two different Mn2+ (Band at 495 & 512nm), Fe3+ (Band at 670nm) and S2- (Band at 603nm) emission centers.
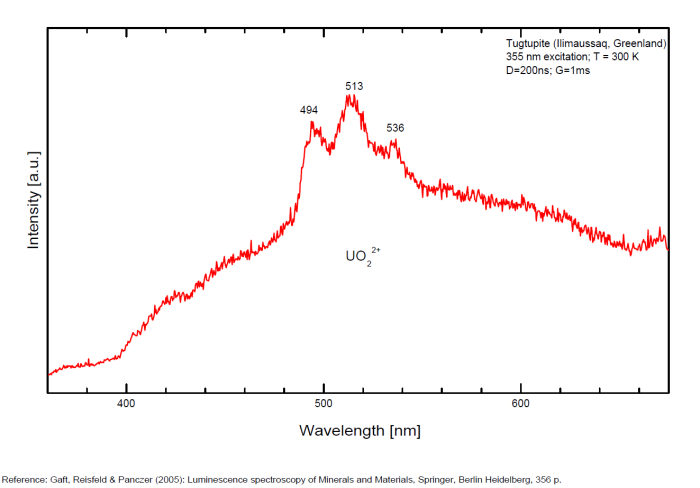
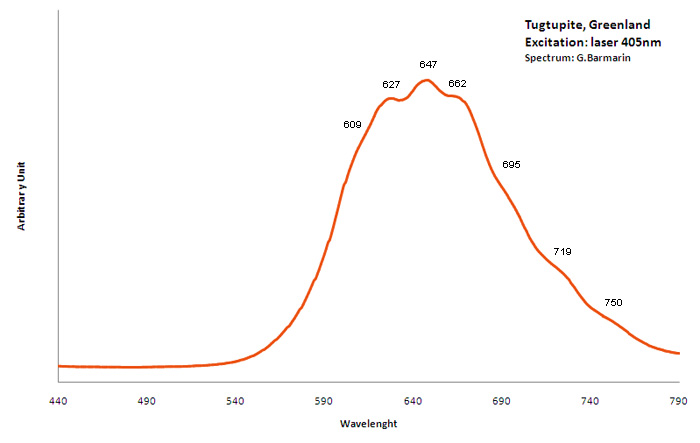
URANOCIRCITEBa(UO2)2(PO4)2 12H2O
Activator(s):
(UO2)2+ (ion Uranyle) intrinsèque ,
Peaks in the spectrum (nm):
[ 488.1, 503.5 523.9, 547, 572.1, 599.7nm ] [ 503, 525, 548, 573, 601, 630nm (échantillon en collection) ]
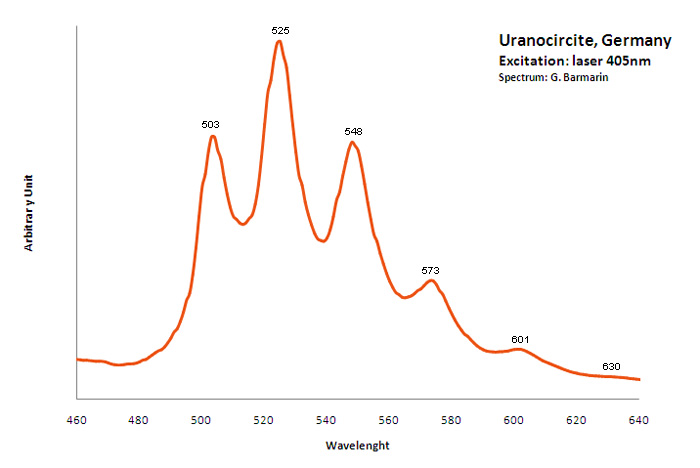
URANOPHANECa(UO2)2[SiO3(OH)]2 5H2O
Activator(s):
(UO2)2+ (ion Uranyle) intrinsèque ,
Peaks in the spectrum (nm):
499nm, 513nm, 535nm, 560nm, 586nm, 597nm, 609nm
Comments on spectra and activators:
Spectra Gorobets: 19400 cm-1, 18670 cm-1, 17960 cm-1;
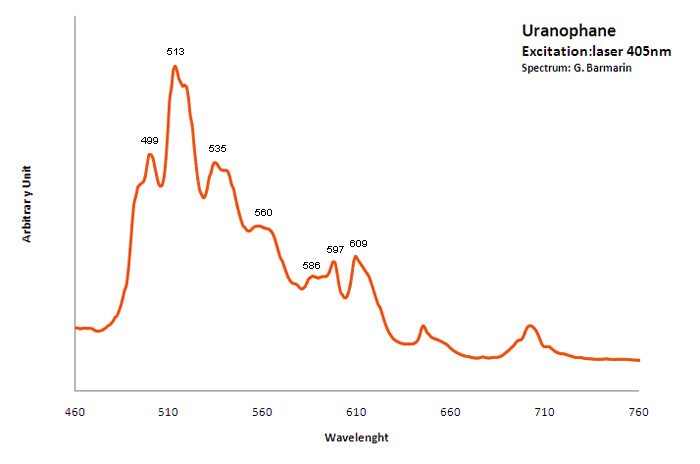
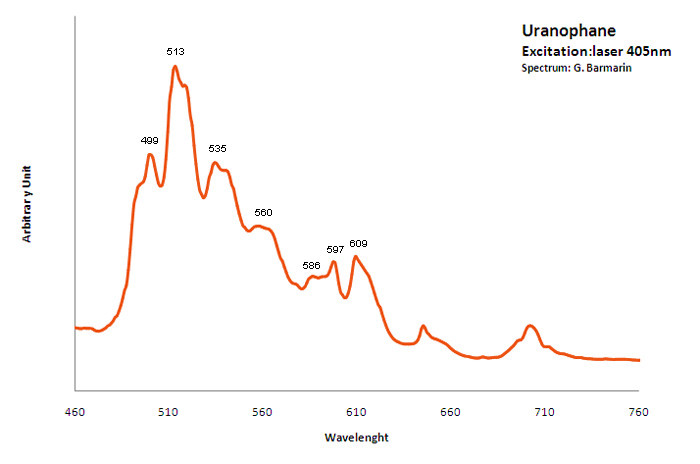
URANOPILITE(UO2)6(SO4)O2(OH)6 14H2O
Activator(s):
(UO2)2+ (ion Uranyle) intrinsèque ,
Peaks in the spectrum (nm):
(UO2)2+ : (514nm), 528nm, 550nm, (576nm), (604nm), (642nm)
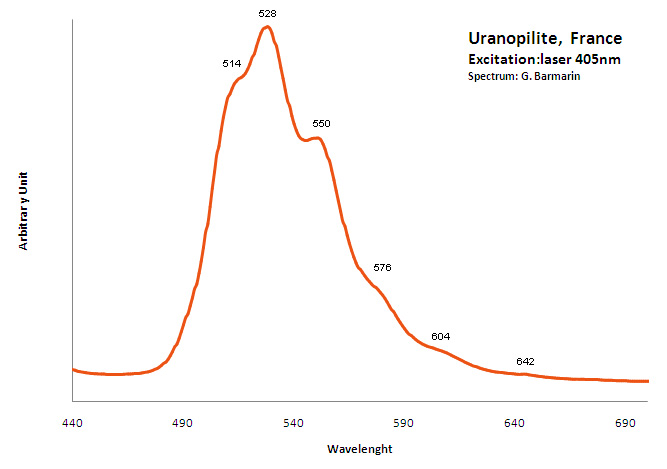
URANOSPATHITEAl0,5-1(UO2)4(PO4)4 20-21H2O F<1
Activator(s):
(UO2)2+ (ion Uranyle) intrinsèque ,
Peaks in the spectrum (nm):
(UO2)2+ : 500nm, 521nm, 545nm, 571nm, 599nm, 630nm

URANOSPINITECa(UO2)2(AsO4)2 10H2O
Activator(s):
(UO2)2+ (ion Uranyle) intrinsèque ,
Peaks in the spectrum (nm):
(UO2)2+ : 488.2, 502.2, 523.6, 547.2, 573.0, 600.7 501nm, 522nm, 546nm, 570nm, 597nm
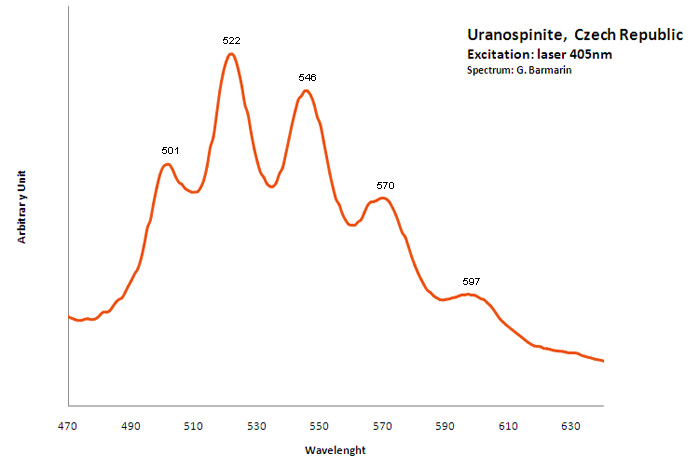
USSINGITENa2AlSi3O8(OH)
Activator(s):
(UO2)2+ (ion Uranyle) en impureté,
Peaks in the spectrum (nm):
(UO2)2+ : 498nm, 518nm, 539nm, 563nm
Comments on spectra and activators:
Typical spectrum of Uranyl impurities.
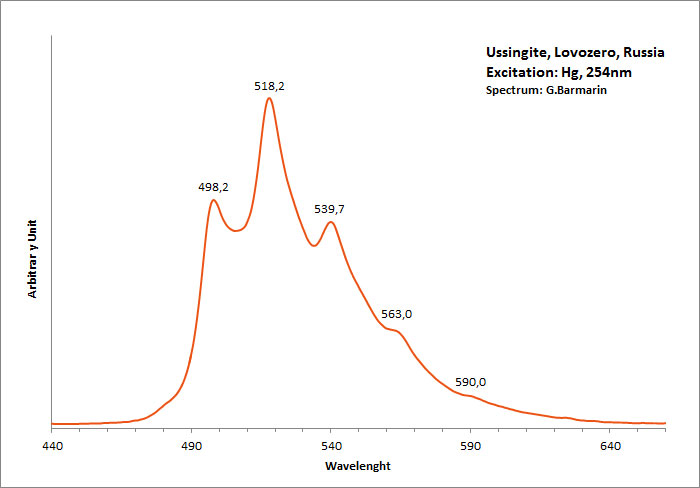
Excitation: Superbright 254nm. Col. G. Barmarin; Spectre: G. Barmarin
valchovite/ (polymeric sesquiterpen alcohol)
Activator(s):
Matière organique intrinsèque,
Peaks in the spectrum (nm):
broad band centered around 600nm with curve modification at 570nm, 603nm and 654nm
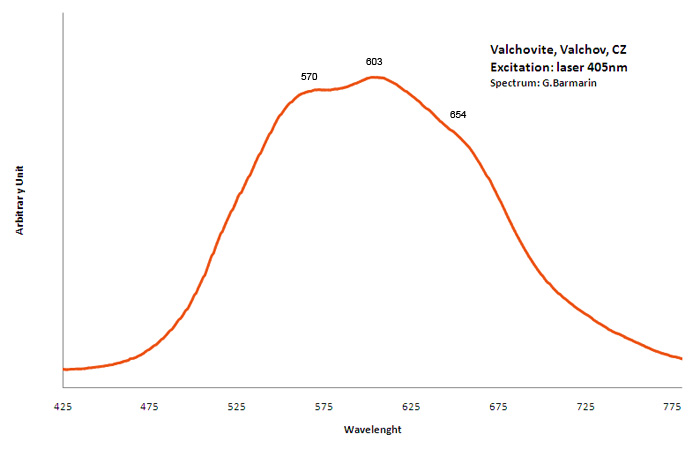
VANDENBRANDEITECu+2(UO2)(OH)4
Activator(s):
(UO2)2+ (ion Uranyle) intrinsèque ,
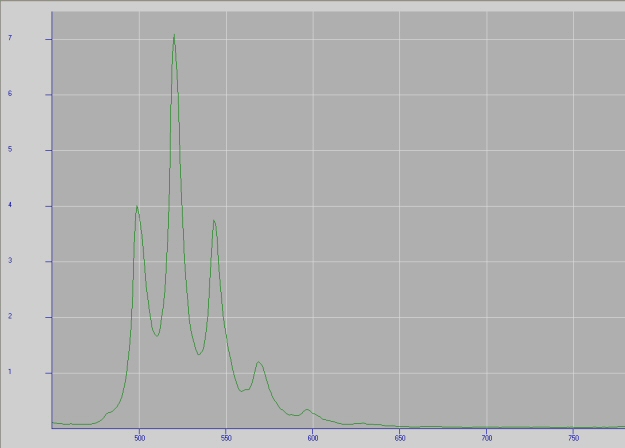
VLASOVITENa2ZrSi4O11
Activator(s):
n[TiO6] cluster,
Other activators:
(UO2)2+ (ion Uranyle) en impureté,
Peaks in the spectrum (nm):
Very large band with max at 550 nm (Gorobets) UO22+ : 502nm, 518nm, 539nm, 560nm (sample in collection)
Comments on spectra and activators:
Blue luminescence due to TiO6 (Gaft)
The specified directory does not exist.
WALSTROMITEBaCa2Si3O9
Activator(s):
Mn2+ ,
Other activators:
Eu2+, Nd3+, Tm3+,
Peaks in the spectrum (nm):
Comments on spectra and activators:
Time-resolved luminescence spectra are characterized by Tm3+, Eu2+ and Mn2+ centers (Gaft et al. 2013). Excitation by CW laser with 532 and 785 nm revealed several luminescence bands and lines supposedly connected to Mn2+ and Nd3+
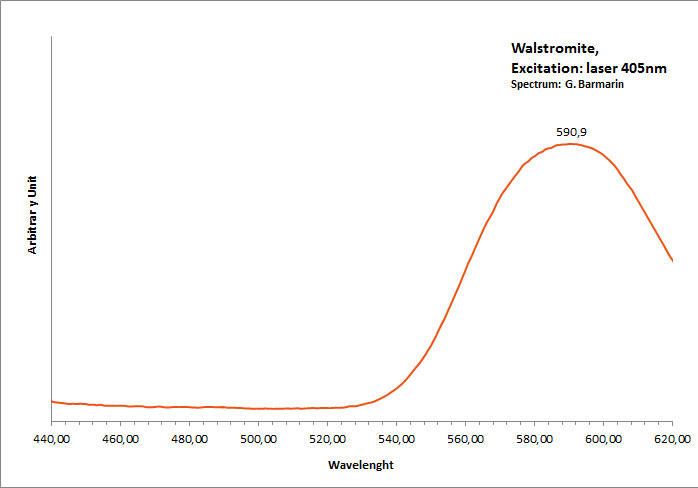
WEEKSITE(K,Ba,Ca)2(UO2)2Si5O13 H2O
Activator(s):
(UO2)2+ (ion Uranyle) intrinsèque ,
Peaks in the spectrum (nm):
UO22+: 532, 550nm (LW), 511, 531, 553nm (SW)
Comments on spectra and activators:
The peaks of the spectrum are not very well defined but there is clearly a peak around 532nm and a second one at 550nm. Other peaks should be found after deconvolution of the spectrum but are only slightly visible as shoulders in the curve.
wernerite/ (voir aussi/see also MARIALITE)
Activator(s):
S2-,
Peaks in the spectrum (nm):
(S2)- : Very broad band peaking around 600-603 nm with some waves superposed
Comments on spectra and activators:
De Ment studied the fluorescence spectrum of wernerite in 1943. The emission spectrum is dependent upon excitation wavelength, indicating that S2- occupies several different sites. The luminescence emission spectrum is peculiar in that it is a series of distinct, nearly equally spaced bands, covering the region from 500 to 700nm with maximum intensity just below 600nm. This luminescence was originally attributed to 2+ UO 2 . Kirk (1954; 1955) showed that the luminescence center was more likely to be a polysulfide ion, S,, and later (Schulman and Kirk, 1964) deduced it to be S 2. Similar luminescence is found in hackmanite and in sodium thiosulfate reduced at 900 ° C. under UV light. The luminescence of wernerite can be enhanced by heating the samples to 900 °C for 24 h. (see Burgner R, Scheetz B, White W (1978) Vibrational structure of the S2- luminescence in scapolite. Phys Chem Miner 2:317–324).
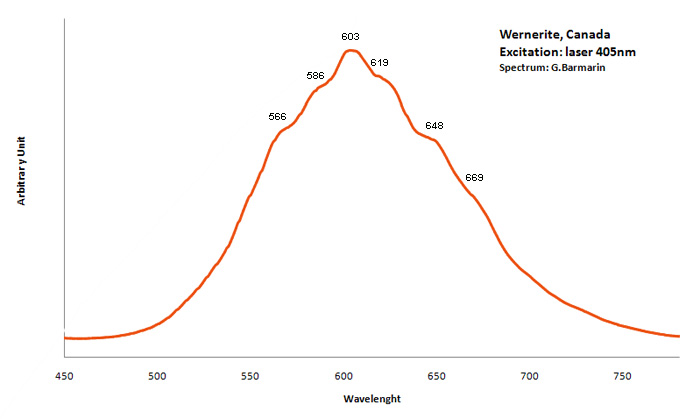
WHEWELLITECaC2O4 H2O
Activator(s):
ST (Singlet-triplet)-Matière organique en impureté,
Peaks in the spectrum (nm):
410nm, 480nm, 512nm 546nm 604nm 708nm
Comments on spectra and activators:
Whewellite is often polluted by organic compound probably causing the fluorescence and phosphorescence (Humic acid? polycyclic aromatic hydrocarbons?).
Excitation: laser 405nm. Col. G. Barmarin; Spectre: G. Barmarin
WILLEMITEZn2SiO4
Activator(s):
Mn2+ ,
Peaks in the spectrum (nm):
Mn2+ replacing Zn2+ : broad band peaking at +/- 525nm
Comments on spectra and activators:
Lifetime: 400μs @520nm The action of ultra-violet rays on a large series of minerals of all kinds was tested by G. F. Kunz and C. Baskerville in 1903, and by E. Engelhardt in 1912. T. Liebisch in 1912 determined spectroscopically the nature of the green fluorescence from willemite. W.S. Andrews has pointed out in 1922 that artificially prepared willemite is only active when it contains some manganese, the shade of the green fluorescence depending on the amount of manganese present. Palache (1928) confirmed that the willemite containing manganese fluoresces the strongest. Dake (1941) stated that most connoisseurs of fluorescent minerals agree that willemite from Franklin an Ogdensburg, N.J. is the most spectacular of all luminescent minerals.

WITHERITEBaCO3
Activator(s):
,
Other activators:
Eu2+,
Peaks in the spectrum (nm):
Eu2+ replacing Ba2+ : Peak at 390nm peak at 480 nm (unknown)
Comments on spectra and activators:
Witherite was studied by steady-state luminescence spectroscopy and trivalent REE, such as Gd, Dy and Eu, have been found (Gorobets and Rogojine 2001). Excitation by CW laser with 532 and 780 nm revealed several luminescence lines and one broad band. IR lines under 780 nm excitation may be evidently ascribed to Nd3+. The band is somewhat similar to orange emission of barite ascribed to Ag+ luminescence center. (Gaft)
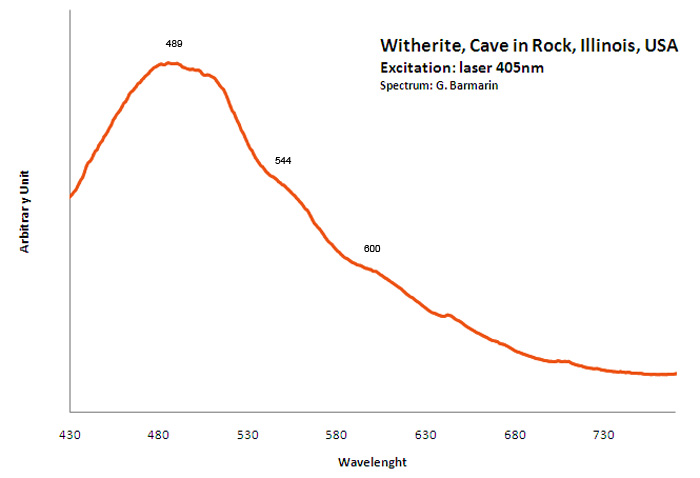
WOLLASTONITECaSiO3
Activator(s):
Mn2+ ,
Other activators:
Cr3+, Fe3+,
Peaks in the spectrum (nm):
Mn2+I repl. Ca2+I : 555-560nm Mn2+II repl. Ca2+II : 580nm Mn2+III repl. Ca2+III : 603, 640nm Fe3+ repl. Si4+ : 690-700nm Cr3+ : narrow R-lines at 720nm + 794 - 840nm broad band Cr3+II : 794nm Cr3+III : 840nm
Comments on spectra and activators:
Steady-state luminescence of wollastonite has been previously studied and luminescence of Mn2+, Fe3+ and supposedly Cr3+ has been proposed (Min’ko et al. 1978). (Gaft)
The specified directory does not exist.
youngite
Activator(s):
(UO2)2+ (ion Uranyle) en impureté,
Peaks in the spectrum (nm):
UO22+ : 499nm, 522nm, 544nm 567, 594, 601nm (?)
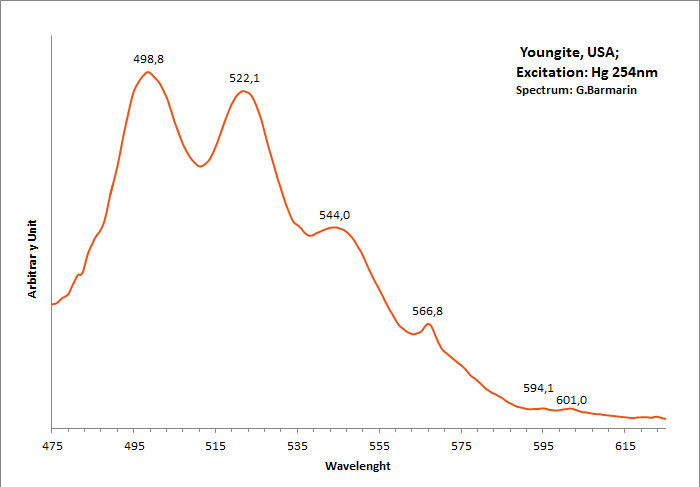
ZEKTZERITENaLiZrSi6O15
Activator(s):
Ti - O,
Peaks in the spectrum (nm):
Ti-O : large band with max at 490 nm (Gorobets) 490, 513, 545, 601nm (sample in collection)
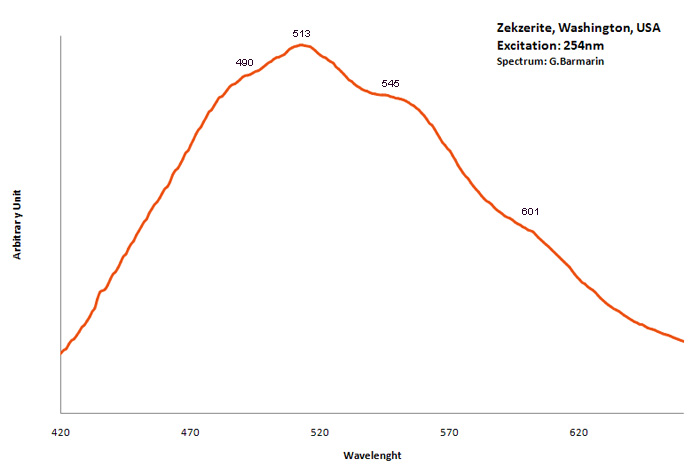
Col. G. Barmarin; Spectre: G. Barmarin
ZEUNERITECu(UO2)2(AsO4)2 12H2O
Activator(s):
(UO2)2+ (ion Uranyle) intrinsèque ,
Peaks in the spectrum (nm):
503, 525, 547, 575
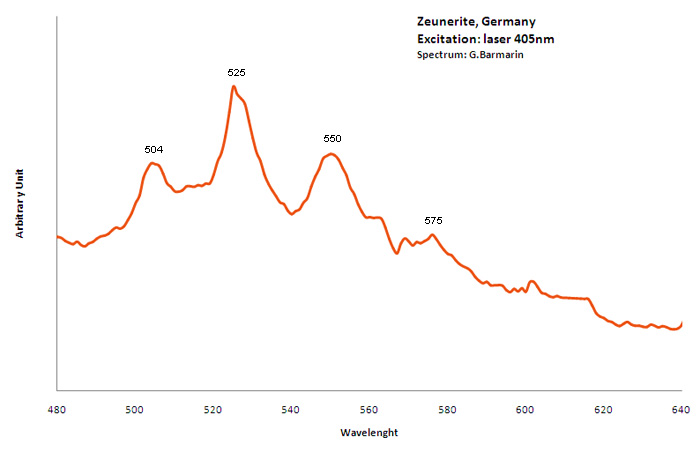
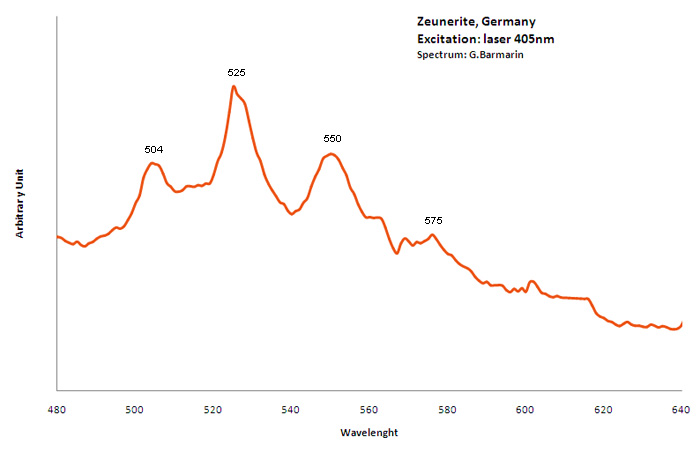
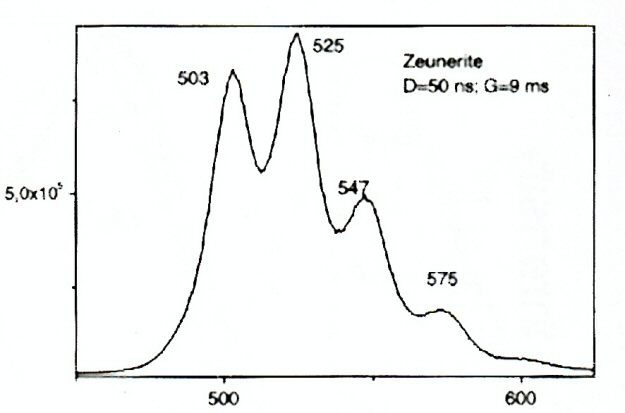
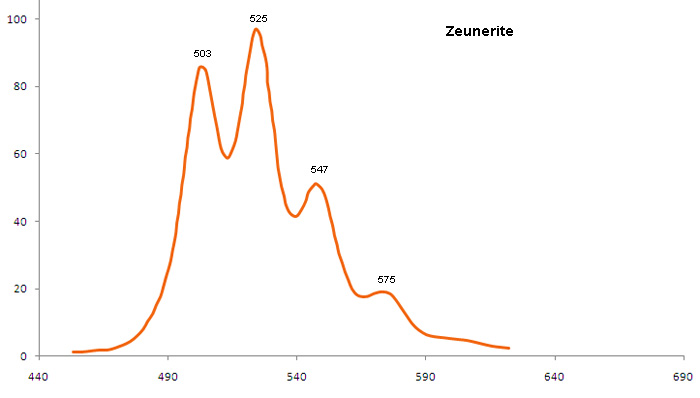
ZIPPEITEK (UO2)2(SO4)(OH)3 H2O
Activator(s):
(UO2)2+ (ion Uranyle) intrinsèque ,
Peaks in the spectrum (nm):
505, 527, 551, 577
Comments on spectra and activators:
Lifetime: 3μs @ 540nm;
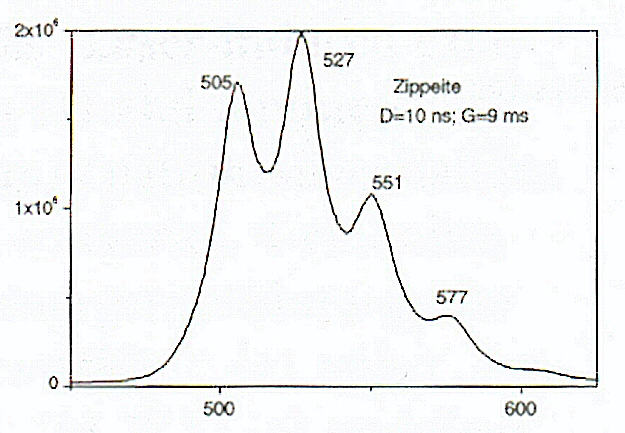
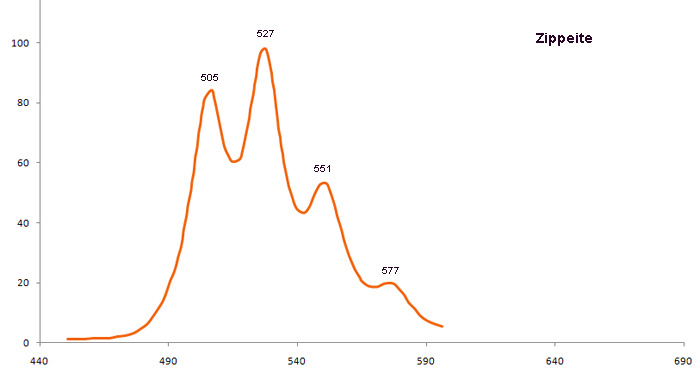
ZIRCONZrSiO4
Activator(s):
Centres dûs aux effets des radiations,
Other activators:
(UO2)2+ (ion Uranyle) en impureté, Cr3+, Fe3+, Sm3+, Eu3+, Dy3+, Ho3+, Er3+, Tb3+, Nd3+, Yb3+, Tm3+,
Peaks in the spectrum (nm):
Dy3+ : 472, 481, 486nm (UO2)2+ : 506, 523, 550nm Fe3+ : 750 nm, Δ = 110–120 nm and τ = 3–5 ms Neutron and alpha irradiation : 575 nm, peak half-width (Δ) = 120–130 nm, and decay time (τ) = 30–35 µs
Comments on spectra and activators:
The crystal chemistry of zircon strongly favors the incorporation of REE in Zr4+ site. The REE impurities become luminescent in this crystallographic environment. The steady-state luminescence in natural zircons is dominated by broad emission arising from radiation induced centers and narrow emission lines of Dy3+ (Trofimov 1962; Tarashchan 1978). These emissions obscure the spectra of other REE. The thermal treatment enables to solve this problem in certain cases using the fact that the intensity of broad band luminescence quickly decreases after heating at 700 °C–800 °C, while the intensities of the REE lines remain nearly constant (Shinno 1986, 1987). Even after heating the samples not all the REE can be identified by steady-state spectroscopy since the weaker luminescence lines of certain REE are obscured by stronger luminescence of others. For example, the luminescence of Pr3+ is difficult to detect because the lines of Sm3+, Dy3+ and Nd3+ hide its radiative transitions. In turn, Tb3+ conceals luminescence of Tm3+ and so on. Different suppositions are made in previous studies and even the question about yellow luminescence connection with intrinsic or impurity defect remains open. The yellow band with peak wavelength (λmax) = 575 nm, peak half-width (Δ) = 120–130 nm, and decay time (τ) = 30–35 µs is connected with neutron and alpha irradiation. Activators: broadband emission from radiation induced centers and lines from Dy3+ (Trofimov in Gaft) masking other lines from REE in Zr4+ site.
The red band with λmax = 750 nm, Δ = 110–120 nm and τ = 3–5 ms is connected with Fe3+. (M. Gaft, I. Shinno, G. Panczer and R. Reisfeld, Laser-induced time-resolved spectroscopy of visible broad luminescence bands in zircon, Mineralogy and Petrology, vol76, 2002)
The intensity of the broadband luminescence quickly decrease after heating at 700-800° while the lines from REE stay constant (Shinno in Gaft).
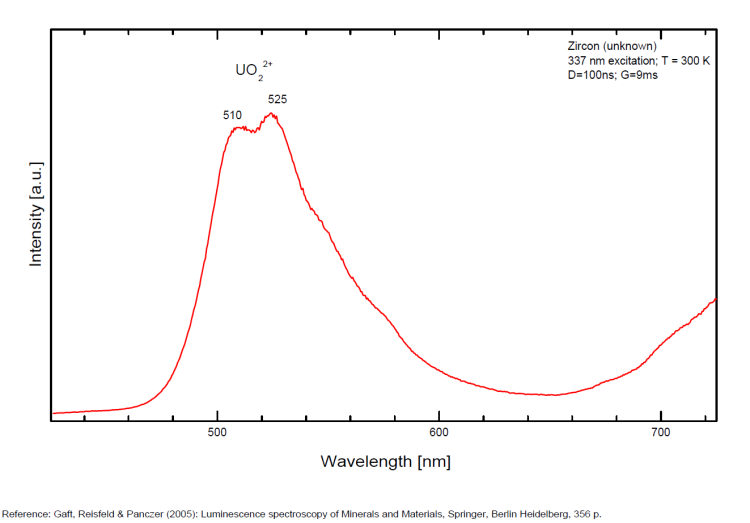
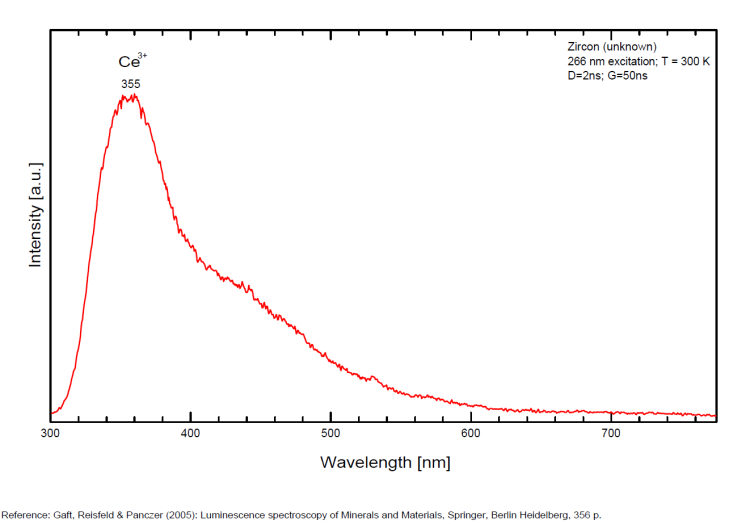
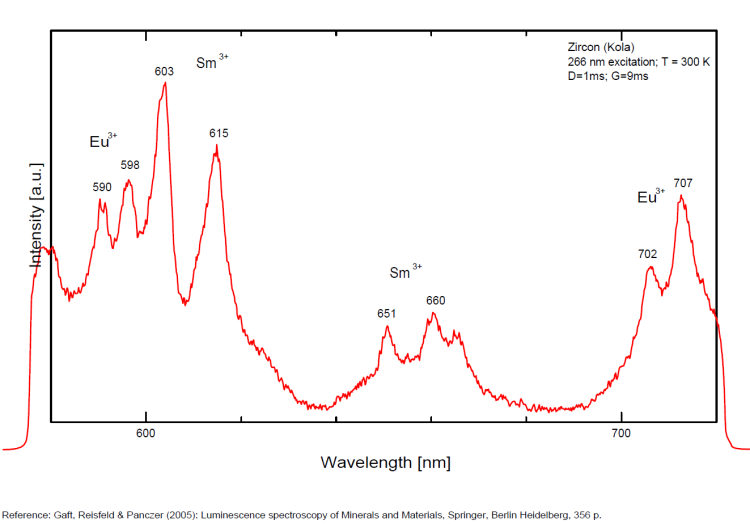
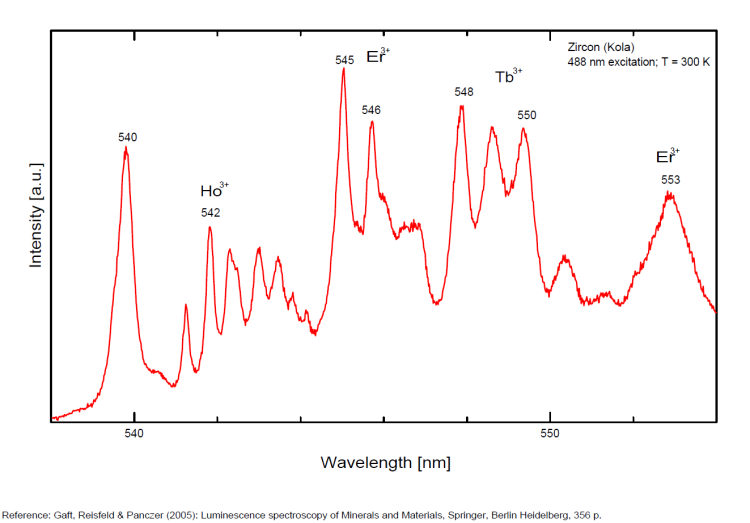
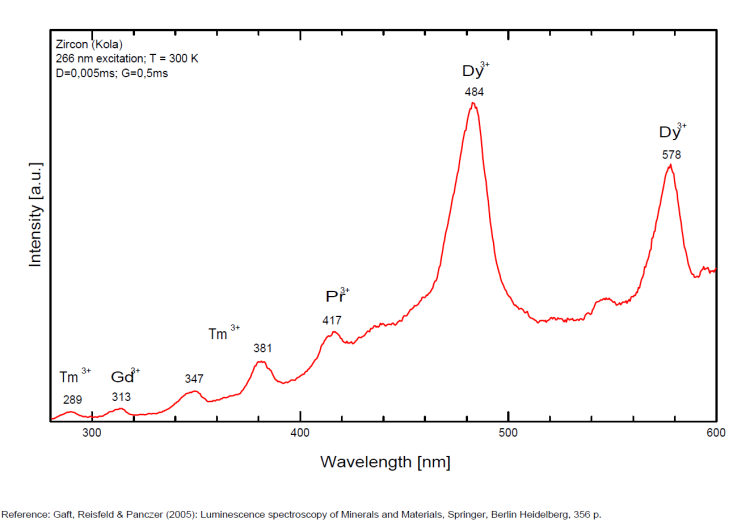
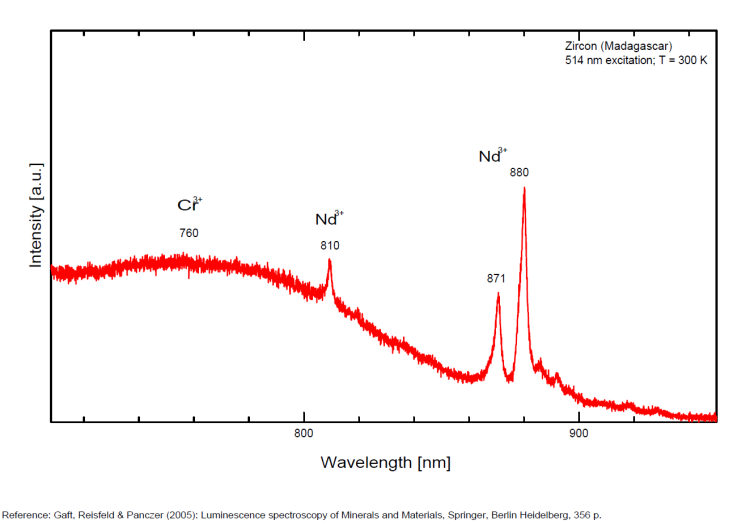
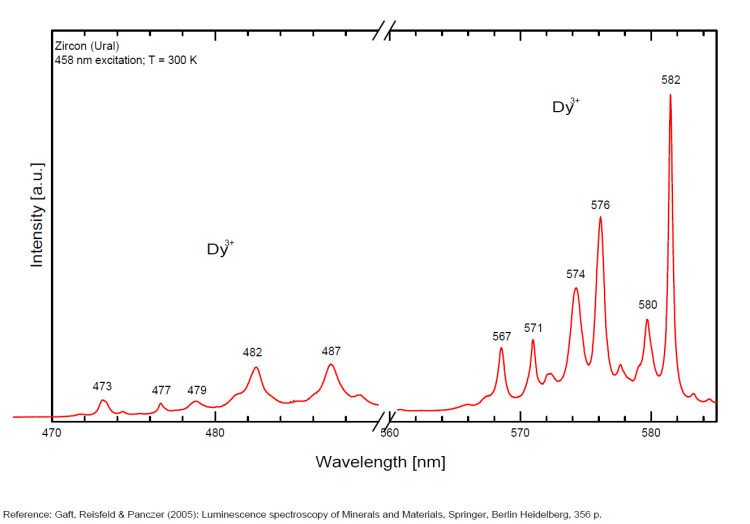
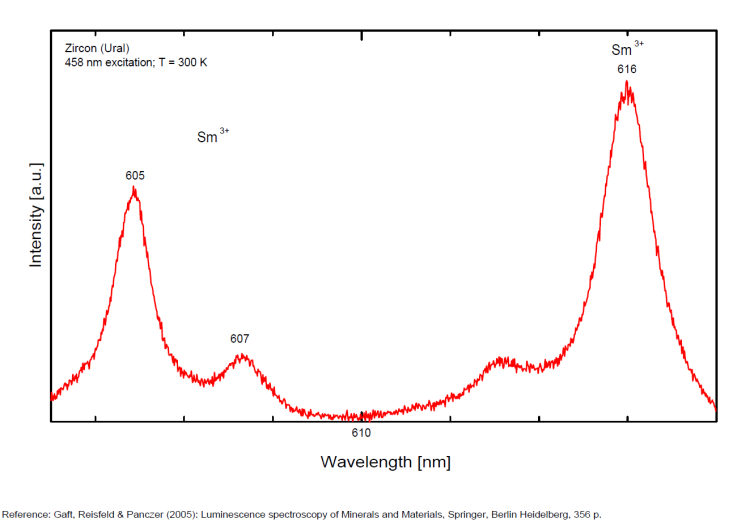
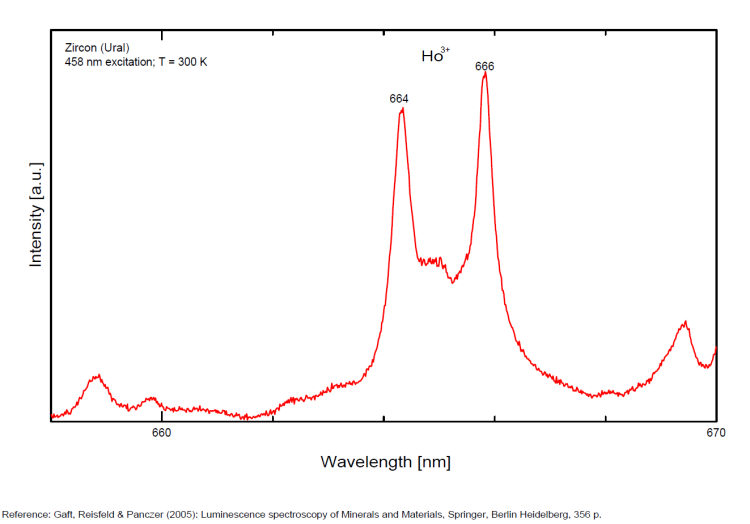
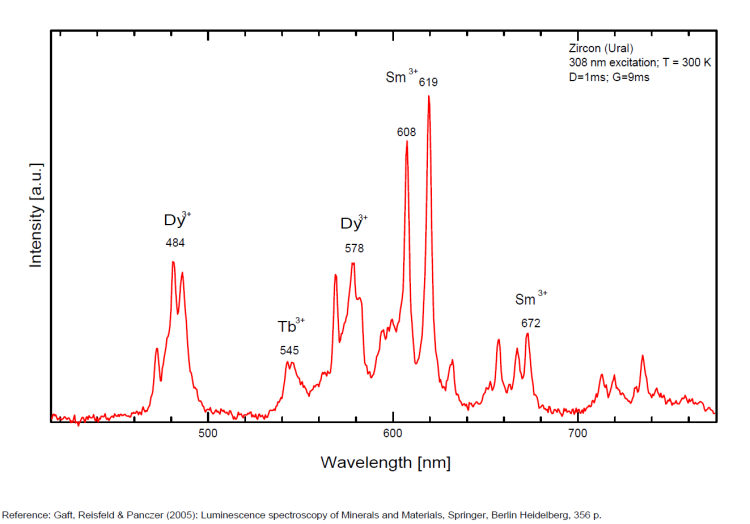
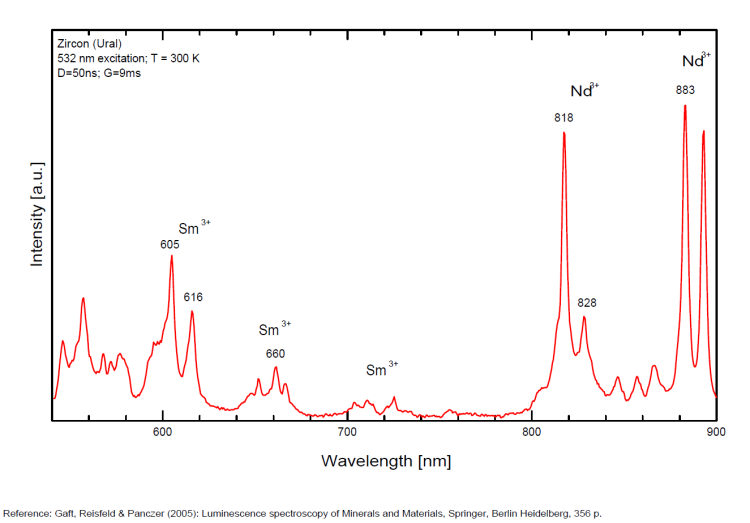
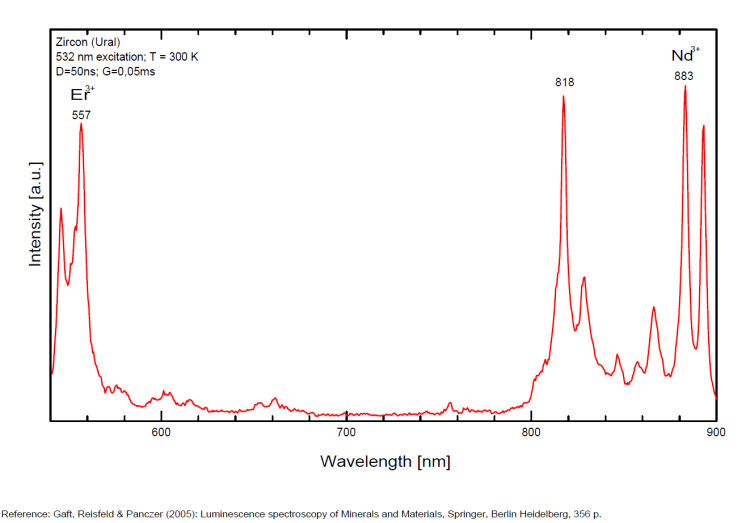
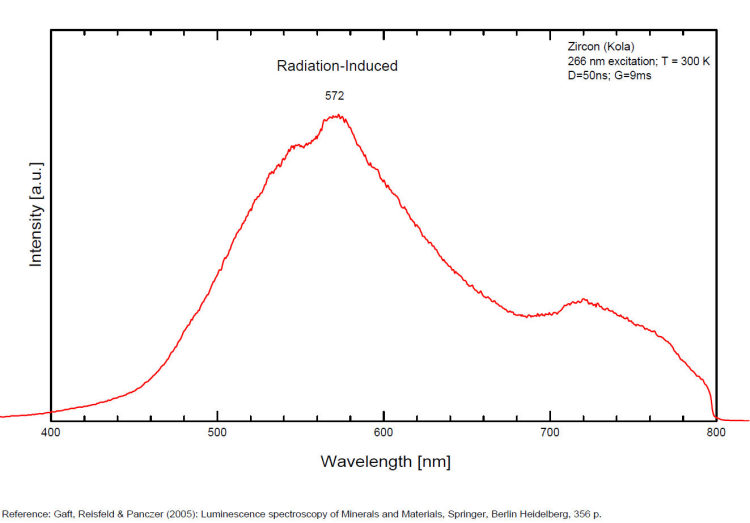
ZNUCALITECaZn11(UO2)(CO3)3(OH)2O 4H2O
Activator(s):
(UO2)2+ (ion Uranyle) intrinsèque ,
Peaks in the spectrum (nm):
(UO2)2+ : 469nm, 487nm, 508nm, 530nm, 554nm, 581nm
ZOISITECa2Al3(SiO4)3(OH)
Activator(s):
V2+,
Other activators:
Eu2+, Dy3+, Tb3+, Mn2+ , V2+,
Peaks in the spectrum (nm):
Eu2+ : 440nm Dy3+ : 480, 575, 578nm Tb3+ : 544nm V2+ : 700, 705, 718, 726 nm