Database of luminescent minerals
GROSSULAR (French name: GROSSULAIRE )
Chemical formula: Ca3Al2(SiO4)3
Family: Silicates
Status: IMA-GP
Crystal system : Isometric
Display mineral: NON
Associated names (luminescent varieties, discredited names, synonyms, etc.): grenat, hessonite, hydrogrossulaire, tsavorite,
Luminescence:
Longwave UV (365nm) colors: |
Red , Bluish White , Violet red , Pink , Salmon pink , | ||
Intensity LW:Weak | |||
Midwave UV (320nm) colors: |
Red , Bluish White , Violet red , | ||
Intensity MW:Weak | |||
Shortwave UV (254nm) colors: |
Red , Yellowish White , Orange , Dark Orange /Tawn , | ||
Intensity SW:Extremely weak | |||
Daylight picture

GROSSULAIRE Lake Jaco, Chihuahua Mexico;
Col. G.Barmarin; Photo: G. Barmarin
Longwave (365nm) picture

GROSSULAIRE Lake Jaco, Chihuahua Mexico; UVLW
Col. G.Barmarin; Photo: G. Barmarin
Shortwave (254nm) picture

GROSSULAIRE Lake Jaco, Chihuahua Mexico; UVSW
Col. G.Barmarin; Photo: G. Barmarin
Do you have a photo of this mineral you would like to see in the gallery? Contact us!
Phosphorescence (in the common sense of the term) observable with the naked eye:
No phosphorescence visible to the naked eye under any type of UV
Thermoluminescence: OUI
Comments:
Grossular garnet from Lake Jaco, Chihuahua Mexico, may be found as dark cherry red crystals, with vesuvianite. Under either MW or a filtered high pressure UV source, these garnets fluoresce brilliantly in a pure red. Mont-Saint-Hilaire: grossular OH-bearing (hibschite)
Activator(s) and spectrum:
Activator(s): Cr3+, Mn4+, Mn2+ , V2+,
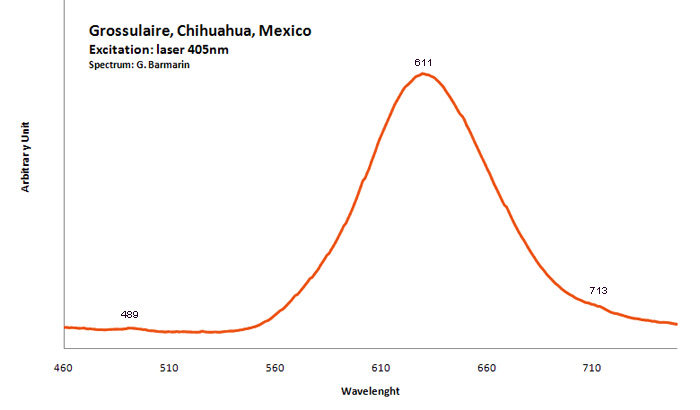
Peaks in the spectrum (nm):
Broad band peaking at 611nm, peak at 489nm and 713nm (échantillon en collection) Cr3+ : Lines at 690, 694, 698 and 707nm Mn2+ substituting to Ca2+ : band around 590 -605 nm Mn3+ : broad band at 653nm Mn4+ : broad band at 670nm V2+ : peak at 717 nm (Gaft)
Col. G.Barmarin; Photo: G. Barmarin
Spectrum Galery:

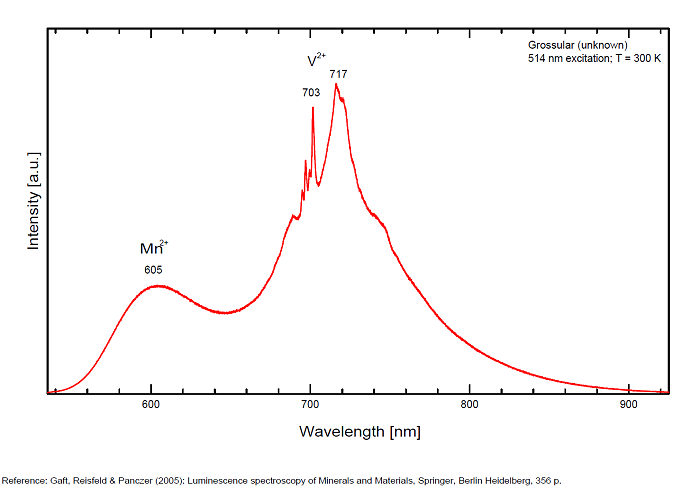 ...
...Comments on spectrum and activators:
The color is identical to that of red-fluorescing ruby corundum or spinel. As with those minerals, chromium in place of aluminum is the likely activator. Other activator: V2+ (717 nm), Nd3+ (462, 476, 482,501 nm) and possibly Mn2+ band around 605-610 nm (decay time: several ms). Natural grossular luminescence was studied by continuous wave (CW) luminescence technique and has been connected with Cr3+ centers, which substitutes for Al3+ and occupies a site with distorted octahedral symmetry (which in fact is trigonal C3i). Trivalent chromium in grossular occupies high crystal field position. The luminescence spectra of Cr3+ in grossular at room temperature contain a strong broad band peaking at 720 nm and three sharp lines centered at 697, 700 and 701 nm. Excitation by CW laser with 785 nm revealed IR luminescence lines which evidently may be ascribed to trivalent REE, such as Pr, Ho, Nd, Er and Yb (Gaft).
Best localities for fluorescence (*):
- Lake Jaco, Chihuahua Mexico;
- Jeffrey Mine, Asbestos, Les Sources RCM, Estrie, Québec, Canada (pink under LW);
- Lelatema Mountains, near Arusha, Tanzania (colorless crystal luminescent in strong apricot color under SW);
- variety hessonite from Beaver Mine, Thetford Mine, Quebec, Canada (bright orange under SW);
- King mine, Thetford Mines, Les Appalaches RCM, Chaudière-Appalaches, Québec, Canada (orange SW);
- Sierra de Cruces, Mun. de Sierra Mojada, Coahuila, Mexico;
(*)The data are not exhaustive and are limited to a few remarkable localities for fluorescence
Bibliographic reference for luminescence:
- The Henkel Glossary of Fluorescent Minerals, Dr. Gerhard Henkel, Published by the FMS, 1989 ,
- Fluorescence: Gems and Minerals Under Ultraviolet Light, Manuel Robbins, 1994, Geoscience Press, ISBN 0-945005-13-X ,
- Luminescence Spectroscopy of Minerals and Materials, M. Gaft, R. Reisfeld, G. Panczer, Springer Editor, ISBN: 10 3-540-21918-8 ,
Reference for luminescence on the Internet:
- MONT-SAINT-HILAIRE, History, Geology, Mineralogy, Laszlo HORVATH, The Canadian Mineralogist, Special Publication 14, 2019
Images:
- Jeffrey Mine, Asbestos, Québec, Canada: http://www.mindat.org/photo-698392.html
- King mine, Québec, Canada: http://www.mindat.org/photo-240464.html
Mineralogical reference on the Internet:
 http://www.mindat.org/show.php?name=Grossular
http://www.mindat.org/show.php?name=Grossular
 http://webmineral.com/data/Grossular.shtml
http://webmineral.com/data/Grossular.shtml
Internet Search:
 Image search on 'Google Images'
Image search on 'Google Images'
 Search for documents in all languages on Google
Search for documents in all languages on Google
A request providing no result means only that no such reference exists in the database, but it does not mean that what you are looking for does not exist, just not to our knowledge. If you think you have found an error or omission, please let us know via the contact page being sure to cite the source of information.