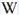Database of luminescent minerals
BENITOITE
Chemical formula: BaTiSi3O9
Family: Silicates
Status: IMA-GP
Crystal system : Hexagonal
Display mineral: OUI
Luminescence:
Longwave UV (365nm) colors: |
Red , | ||
Intensity LW:Weak | Frequency LW:Rarely | ||
Shortwave UV (254nm) colors: |
Bluish White , | ||
Intensity SW:Very Strong | Frequency SW:Always | ||
Daylight picture
Longwave (365nm) picture

BENITOITE, UVLW ;
Photo and Copyright: G. Barmarin
Shortwave (254nm) picture

BENITOITE;
UVSW
Photo and Copyright: James Hamblen
Site of the author
Used with permission of the author
Pictures Galery:



 ...
...  Go to the galery (42 pictures)
Go to the galery (42 pictures)
Do you have a photo of this mineral you would like to see in the gallery? Contact us!
Phosphorescence (in the common sense of the term) observable with the naked eye:
Type d'UV |
Couleur |
Intensité |
Fréquence d'observation |
|---|---|---|---|
UV longs (365nm): | None by naked eye | UV courts (254 nm): | None by naked eye |
Activator(s) and spectrum:
Activator(s): TiO6, Cr3+, Fe3+, Ti3+, Ti4+, Cu+, Mn4+, Ti - O,
Peaks in the spectrum (nm):
TiO6 : broad band at 419nm (due to the transition of 3T1 → 1A1 in the TiO6 octahedra)
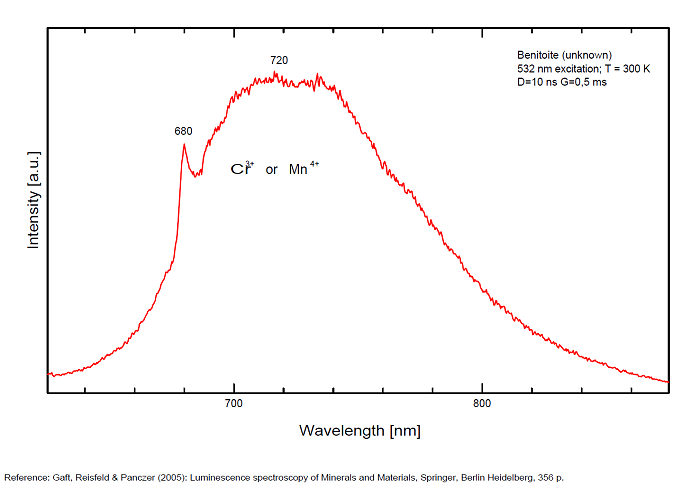
Ti3+ : broad band around 650-660nm with half-width of +/-135nm
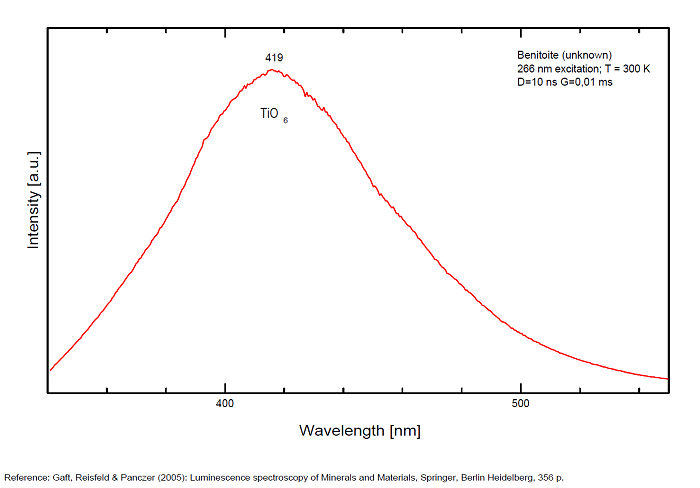
Cr3+ : peak at 680nm
Mn4+ : large band at 720nm
Cu+? replacing Ba2+ and Ti3+? (exc 337nm:(Gorobets)): 720nm
450nm (exc 254nm)
Spectrum: Michael Gaft, Petah Tikva, Israel. Plot: Institute of Mineralogy, University of Vienna, Austria, with permission of the authors.
Spectrum Galery:

 ...
...Comments on spectrum and activators:
Robbins cites Fe as activator for the red color and Ti for the blue; recent spectrographic studies give new data: Decay time shortening and thermal quenching are connected with nonradiative decay within the TiO6 luminescence center, while energy migration does not take place at least up to room temperature.
There are three broadbands:
- one around 419 nm (blue band) is connected with intrinsic luminescence centers, namely isolated TiO6 octahedra,
- one at 650 nm (Yellow band) for Ti3+ ,
- one at 720 (half-width 125nm) for Mn4+
and a narrow line at 680 for Cr3+.(Gaft)
Manganese participation is supported by chemical analysis of benitoite (0.03 to 0.11%).
The metastable level 3T1u is the emitting level at low temperatures with a long decay time of 1.1 ms. At higher temperatures an energy level with higher radiation probability must be involved in the emission process, and this level is situated at 0.06 eV higher than the lowest level. These two levels may be connected with 3T1u level splitting or with closely spaced 3T1u and 3T2u levels.
(Source: M. Gaft, L. Nagli, G. Waychunas and D. Weiss, The nature of blue luminescence from natural benitoite, Physics and Chemistry of Minerals, vol31, 2004)
The red emission of benitoite consists of two individual bands and one line and suggest that the activators of luminescence in benitoite system are Ti3+ and a d3 element, namely Cr3+ or Mn4+.
(Source: M. Gaft, L. Nagli, G. Waychunas and G. Panczer, The nature of red luminescence of natural benitoite, Mineralogy and Petrology, Vol85, 2005)
Best localities for fluorescence (*):
- Benitoite Gem Mine, San Benito County, California, USA;
- Big Creek and Rush Creek junction, Fresno County, California, USA;
- Esquire #1 Claim, Rush Creek, Fresno County, California, USA;
- Victor Mine, San Benito County, California, USA;
- Kinzandani, Hashidate, Ohmi, Itoigawa City, Niigata Prefecture, Chubu Region, Honshu Island, Japan;
(*)The data are not exhaustive and are limited to a few remarkable localities for fluorescence
Bibliographic reference for luminescence:
- The Henkel Glossary of Fluorescent Minerals, Dr. Gerhard Henkel, Published by the FMS, 1989 ,
- Fluorescence: Gems and Minerals Under Ultraviolet Light, Manuel Robbins, 1994, Geoscience Press, ISBN 0-945005-13-X ,
- The World of Fluorescent Minerals, Stuart Schneider, Schiffer Publishing, 2006, ISBN 0-7643-2544-2 ,
- Luminescence Spectroscopy of Minerals and Materials, M. Gaft, R. Reisfeld, G. Panczer, Springer Editor, ISBN: 10 3-540-21918-8 ,
- Luminescent Spectra of Minerals, Boris S. Gorobets and Alexandre A. Rogojine, Moscow, 2002 ,
Reference for luminescence on the Internet:
- The nature of blue luminescence from natural benitoite, M. Gaft, L. Nagli, G. Waychunas and D. Weiss, Physics and Chemistry of Minerals, vol31, 2004
- The nature of red luminescence of natural benitoite, M. Gaft, L. Nagli, G. Waychunas and G. Panczer, Mineralogy and Petrology, Vol85, 2005
- Benitoite Gem mine (Mindat.org)
Mineralogical reference on the Internet:
 http://www.mindat.org/show.php?name=Benitoite
http://www.mindat.org/show.php?name=Benitoite
 http://webmineral.com/data/Benitoite.shtml
http://webmineral.com/data/Benitoite.shtml
Internet Search:
 Image search on 'Google Images'
Image search on 'Google Images'
 Search for documents in all languages on Google
Search for documents in all languages on Google
A request providing no result means only that no such reference exists in the database, but it does not mean that what you are looking for does not exist, just not to our knowledge. If you think you have found an error or omission, please let us know via the contact page being sure to cite the source of information.