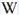Database of luminescent minerals
FLUORITE
Chemical formula: CaF2
Family: Halides
Status: IMA-GP
Crystal system : Isometric
Display mineral: OUI
Associated names (luminescent varieties, discredited names, synonyms, etc.): chlorophane, yttrofluorite, yttrocerite, Tiffany stone, fluorine,
Luminescence:
Longwave UV (365nm) colors: |
Blue , Bluish White , Yellowish White , Pale Yellow , Yellow , Tawn , Red , Violet Pink , Green , Greenish , Violet , Greenish white , Violet blue , Yellowish , | ||
Intensity LW:Very Strong | Frequency LW:Very often | ||
Midwave UV (320nm) colors: |
Blue , Green , | ||
Intensity MW:Medium | Frequency MW:Often | ||
Shortwave UV (254nm) colors: |
Blue , White , Bluish White , Yellowish White , Pale Yellow , Yellow , Tawn , Red , Green , Greenish , Bluish Green , Violet , Greenish white , Violet blue , Yellowish , | ||
Intensity SW:Medium | Frequency SW:Often | ||
Daylight picture

FLUORITE;
Blue Circle Cement Quarry, Eastgate, Weardale, North Pennines, County Durham, England, UK
Photo and Copyright:
Middleearthminerals.com
Used with permission of the author
Longwave (365nm) picture

FLUORITE;
Blue Circle Cement Quarry, Eastgate, Weardale, North Pennines, County Durham, England, UK
UVLW, Photo and Copyright:
Middleearthminerals.com
Used with permission of the author
Pictures Galery:



 ...
...  Go to the galery (35 pictures)
Go to the galery (35 pictures)
Do you have a photo of this mineral you would like to see in the gallery? Contact us!
Phosphorescence (in the common sense of the term) observable with the naked eye:
Type d'UV |
Couleur |
Intensité |
Fréquence d'observation |
|---|---|---|---|
UV longs (365nm): | Greenish white | Strong | Rarely | UV moyens (320 nm): | Greenish white | UV courts (254 nm): | Greenish white | Strong | Rarely |
Triboluminescence: OUI
Thermoluminescence: OUI
Comments:
The classical fluorescing mineral but all fluorites are not luminescent under UV !
CHLOROPHANE variety : green thermoluminescence ;
YTTROFLUORITE variety: SW and LW : yellow, yellowish-white (cream);
Activator(s) and spectrum:
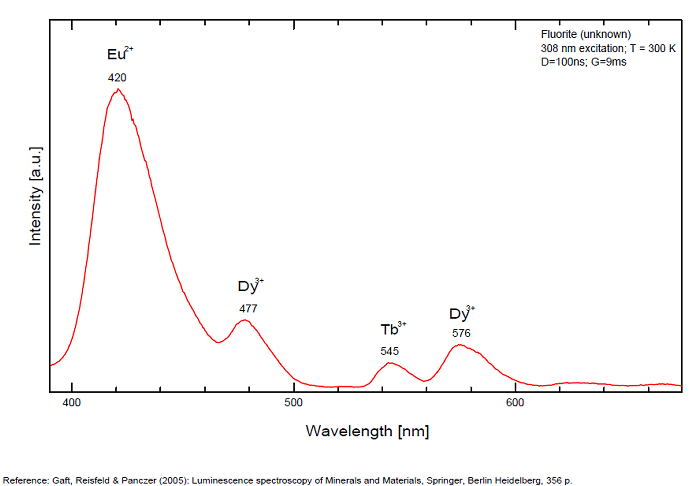
Activator(s): Eu2+, ST (Singlet-triplet)-Matière organique en impureté, Sm2+, Ce3+, Sm3+, Eu3+, Dy3+, Ho3+, Er3+, Tb3+, Pr3+, Nd3+, Yb3+, Tm3+,
Peaks in the spectrum (nm):
Eu2+ repl. Ca2+: 423-425nm (associated with deep blue-violet fluorescence)
Sm2+ repl. Ca2+: 708, 733nm
Yb2+ repl. Ca2+: 550nm
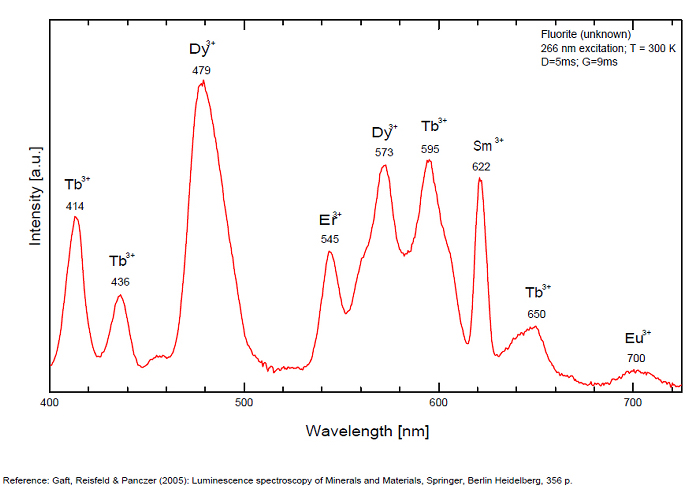
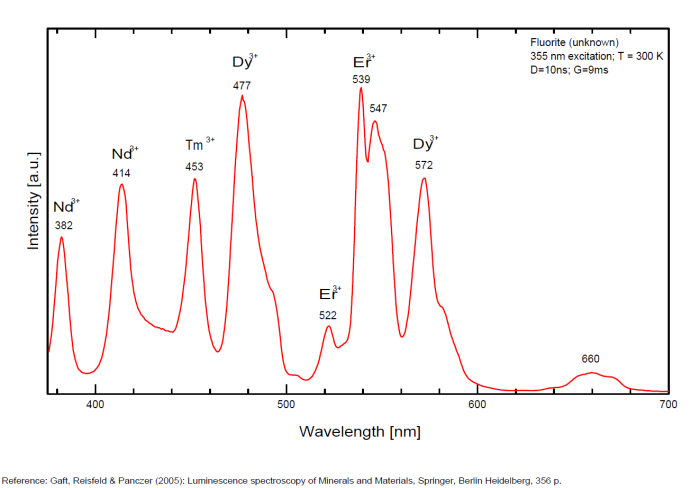
Dy3+:468, 478, 575, 654, 658, 663, 671, 685nm
Tb3+:482, 484, 486, 492, 539, 543, 544, 546, 583, 588nm
Er3+:519, 522, 528, 551, 554nm
Sm3+:561nm
Eu3+: 574, 595, 618, 622, 700nm
Nd3+: 920nm
M-center(2F+Na+): large pic at 720-745 nm
MA center charge stabilized by O2−–F− : 690 nm
MA center charge stabilized by Na+–Ca2+ : 750nm
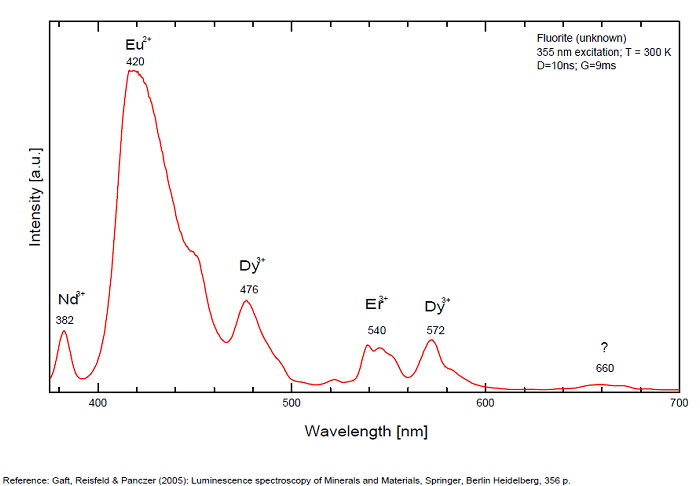
Spectrum: Michael Gaft, Petah Tikva, Israel. Plot: Institute of Mineralogy, University of Vienna, Austria, with permission of the authors.
Spectrum Galery:
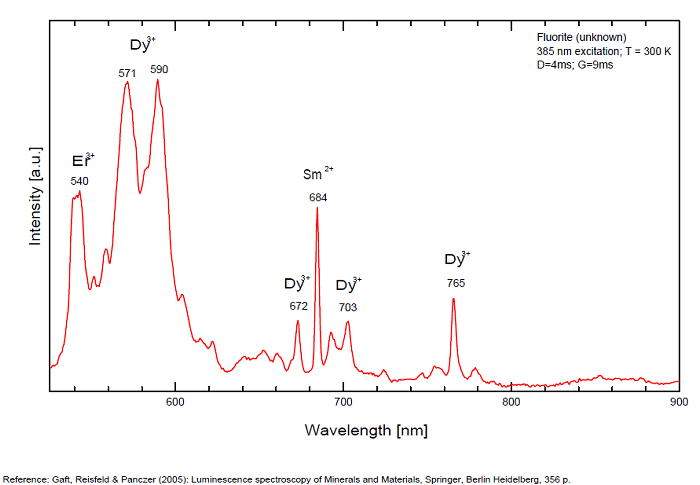
 ...
...Comments on spectrum and activators:
Violet fluo associated with Eu2+ (420-423 nm) with peaks at 320 and 340nm (Ce3+), decay time of Eu2+ is 600–800 ns λex = 415 nm is most suitable for measuring the Ho3+ emission beside the Er3+. Fluorite was one of the first mineral substances being investigated by George G. Stokes in 1852, hence the name fluorescence given at the phenomenon. Fluorite is a reservoir for many of the rare earth elements. As early as 1881, it was pointed out that Cerium was present in fluorite. In 1906, Urbain studied the cathodoluminescence of fluorite and ascribed the cause of fluorescence to RRE (Yttrium, praeseodymium, samarium, dysprosium, europium, terbium and also gadolinium and Ytterbium in chlorophane); Morse (1907), Tanaka (1924), Nichols (1928) and F. G. Wick also investigated the cause of luminescence of fluorite. The fusion of fluorescent fluorite (1100-1200°C) changes the spectrum markedly. Often the blue luminescence spectrum is changed to a more orange-red, showing sharp lines typical of RRE. De Ment makes 2 groups in the luminescent fluorites: group 1: single band in the UV at 300nm and 3 bands in the visible at 475, 508 and 578nm Radiation induced MA centers’ charge stabilized by Na+–Ca2+ is responsible of emission at 750nm and O2−–F− at 690 nm) (Tarashchan 1978, Gaft and Al. 2020).
yellow fluo associated with Dy3+;
M-center: large pic at 720 nm;
Lifetime: 2μs ( @420nm);
group 2: two bands in UV at 280 and 300nm and strong peaks at 475, 510, 536, 548 and 578nm in the visible.
Best localities for fluorescence (*):
- Rogerley Mine, Weardale, County Durham, England;
- Alston Moore, England;
- Dalnegorsk, Primorskiy Kray, Russia;
- Seilles, Andenne, Prov. de Namur, Belgium (LW fluo red);
- Hunza Valley, Gilgit District, Pakistan;
- Braldu Valley, Skardu District, Gilgit-Baltistan, Pakistan (green SW+LW+Phospho);
- Yuno, Shigar District, Gilgit-Baltistan, Pakistan (green SW with fluorapatite in quartz and microcline);
- Mapimi, Durango, Mexico (SW dull red, LW red);
- Hammam-Zriba Mine, Zriba-Village, Zaghouan Governorate, Tunisia (buish-white LW);
- Rosiclare, Illinois, USA;
- Clay Center, Ohio, USA (yellow-white LW + phosphorescence);
- Yuma County, Arizona, USA;
- Fourmile Creek Area, Fremont Co., Colorado, USA (LW blue + SW & MW green)
- Berta Mine, El Papiol, Barcelona, Spain;
- Villabona Mine, Llanera, Asturias, Spain;
- Xianghualing Sn-polymetallic ore field, Linwu Co., Chenzhou, Hunan, China (blue + red fluo LW);
- Sanming, Fujian, China (blue LW with temporary red fluorescence disappearing after a few seconds);
- Taourirt, Taourirt Province, Oriental Region, Morocco (temporary LW red fluorescence disappearing after a few seconds and comming back after a few minutes without UV);
- Aouli, Upper Moulouya mining district, Midelt Province, Drâa-Tafilalet Region, Morocco (yellow cubes SW white, LW blue)
- Kharan District, Balochistan, Pakistan (fluo red LW).
(*)The data are not exhaustive and are limited to a few remarkable localities for fluorescence
Bibliographic reference for luminescence:
- The Henkel Glossary of Fluorescent Minerals, Dr. Gerhard Henkel, Published by the FMS, 1989 ,
- Fluorescence: Gems and Minerals Under Ultraviolet Light, Manuel Robbins, 1994, Geoscience Press, ISBN 0-945005-13-X ,
- The World of Fluorescent Minerals, Stuart Schneider, Schiffer Publishing, 2006, ISBN 0-7643-2544-2 ,
- Luminescence Spectroscopy of Minerals and Materials, M. Gaft, R. Reisfeld, G. Panczer, Springer Editor, ISBN: 10 3-540-21918-8 ,
- Luminescent Spectra of Minerals, Boris S. Gorobets and Alexandre A. Rogojine, Moscow, 2002 ,
- Ultraviolet Light and Fluorescent Minerals, Th. Warren, S. Gleason, R. Bostwick, et E. Verbeek, 1995, ISBN 0-9635098-0-2 ,
- Franklin Website: http://franklin-sterlinghill.com ,
- Luminescenza nel regno minerale, Guido Mazzoleni, fotografia Roberto Appiani, Libri Sandit, 2010, ISBN 978-88-95990-63-7 ,
- Handbook of mineralogy, John W. Anthony, Richard A. Bideaux, Kenneth W. Bladh, and Monte C. Nichols, and published by Mineral Da ,
- The Langesundfjord, history, geology, pegmatites, minerals, Bode Edition, 2010, ISBN 978-3-925094-97-2 ,
- Handbook of Fluorescent Gems and Minerals, a practical guide for the gem and mineral collector, Jack de Ment, 1949 ,
Reference for luminescence on the Internet:
- The Langesundsfjord: history, geology, pegmatites, minerals, Alf Olav Larsen, Bode Verlag Gmbh, 2010 ISBN 978-3-925094-97-2.
- Luminescence properties of Ce3+ and Eu2+ in fluorites and apatites Sabina Bodyl, Mineralogia, 40, No. 1–4: 85–94 (2009)
- Thermoluminescence of Fluorite and Age of Deposition, Frank N. Blanchard, The American Mineralogist, Vol. 51, March-April, 1966
- Luminescence from natural fluorite crystals, Calderon T. , Khanlary M.-R. , Rendell H. M. , Towsend P. D., International journal of radiation applications and instrumentation. Part D. Nuclear tracks and measurements, 1992, vol. 20, no3, pp. 475-485
- Luminescence properties of rare earth ions in fluorite, apatite and scheelite minerals, M. Czaja, S. Bodył, P. Głuchowski, Z. Mazurak and W. Strek, Journal of Alloys and Compounds Volume 451, Issues 1-2, 28 February 2008, Pages 290-292
- The luminescence properties of rare-earth ions in natural fluorite, M. Czaja, S. Bodył-Gajowska, R. Lisiecki, A. Meijerink, Z. Mazurak
- Steady-state Luminescence for qualitative identification of rare-earth ions in minerals, M. Czaja, S. Bodył-Gajowska, Z. Mazurak, 2013, Journal of Mineralogical and Petrological Science
- https://www.mindat.org/article.php/2789/Photoluminescent+Properties+of+the+Fluorites+from+Walworth%2C+New+York
- https://www.mindat.org/article.php/2786/An+Interesting+Fluorite+Occurrence+in+the+Limestone+Capital+of+the+World
- Fourmile Creek Area, Co, USA;
- MONT-SAINT-HILAIRE, History, Geology, Mineralogy, Laszlo HORVATH, The Canadian Mineralogist, Special Publication 14, 2019
- https://www.researchgate.net/publication/345143472_Red_photoluminescence_and_purple_color_of_naturally_irradiated_fluorite
Images:
- Braldu Valley, Skardu District, Gilgit-Baltistan, Pakistan (LW+SW+Phospho): https://www.mindat.org/photo-954531.html
- Yuno, Shigar District, Gilgit-Baltistan, Pakistan (green SW with fluorapatite in quartz and microcline): https://www.mindat.org/photo-629252.html
- Xianghualing Sn-polymetallic ore field, Hunan, China: https://www.mindat.org/photo-933551.html & https://www.mindat.org/photo-933552.html
- Sanming, China (blue LW with temporary red fluorescence disappearing after a few seconds): https://www.mindat.org/photo-932814.html
- Thermoluminescence (Colorado, USA): http://www.mindat.org/photo-291222.html
- Aouli, Morocco: https://www.mindat.org/photo-889701.html
Videos:
- Thermoluminescence:https://www.youtube.com/watch?v=42VGkvXUAvc
- Thermoluminescence (en Français): https://www.youtube.com/watch?v=7bAyKSsLrOQ
- Proton-luminescence et Thermoluminescence of artificial fluorite used in dosimeter: https://www.youtube.com/watch?v=7bAyKSsLrOQ
Mineralogical reference on the Internet:
 http://www.mindat.org/show.php?name=Fluorite
http://www.mindat.org/show.php?name=Fluorite
 http://webmineral.com/data/Fluorite.shtml
http://webmineral.com/data/Fluorite.shtml
Internet Search:
 Image search on 'Google Images'
Image search on 'Google Images'
 Search for documents in all languages on Google
Search for documents in all languages on Google
A request providing no result means only that no such reference exists in the database, but it does not mean that what you are looking for does not exist, just not to our knowledge. If you think you have found an error or omission, please let us know via the contact page being sure to cite the source of information.