Database of luminescent minerals
DOLOMITE
Chemical formula: CaMg(CO3)2
Family: Carbonates
Status: IMA-GP
Crystal system : Rhomboedric
Display mineral: OUI
Luminescence:
Longwave UV (365nm) colors: |
Yellowish White , White , Bluish White , Pinkish White , Orange Red , Red , Violet red , Violet Pink , Pink , Greenish white , Yellowish , | ||
Midwave UV (320nm) colors: |
Yellowish White , Pinkish White , Red , Violet red , Pink , | ||
Shortwave UV (254nm) colors: |
Yellowish White , Bluish White , Pinkish White , Orange Red , Red , Violet red , Violet Pink , Pink , Greenish white , | ||
Daylight picture

DOLOMITE,
Horn Silver Mine, Beaver County, Utah, USA;
Photo and Copyright: James Horste
Used with permission of the author.
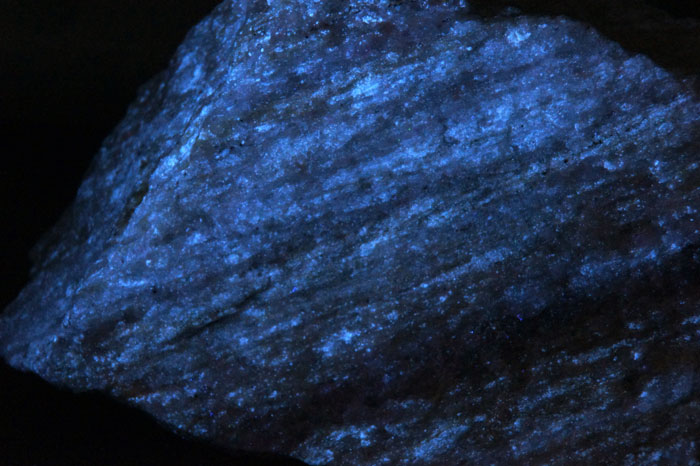
Shortwave (254nm) picture

DOLOMITE,UVSW
Horn Silver Mine, Beaver County, Utah, USA;
Photo and Copyright: James Horste
Used with permission of the author.
Pictures Galery:


 ...
...Do you have a photo of this mineral you would like to see in the gallery? Contact us!
Phosphorescence (in the common sense of the term) observable with the naked eye:
No data
Triboluminescence: OUI
Thermoluminescence: OUI
Comments:
The Crazy Calcite fluo red SW from Franklin, USA is sometime composed by dark red SW fluorescing Dolomite mixed with bright red SW fluorescing Calcite, with black Franklinite and tiny yellow Diopside crystals. The LW response is in shades of red-pink. It is however possible that the fluorescence of dolomite is due to the observation of the phenomenal fluorescence of calcite by transparency.
Activator(s) and spectrum:
Activator(s): Mn2+ , ST (Singlet-triplet)-Matière organique en impureté, Ce3+, Sm3+, Dy3+, Tb3+, Gd3+,
Peaks in the spectrum (nm):
Mn2+I repl. Ca2+ : 578 - 582nm
Mn2+II repl. Mg2+ : 650 - 660nm
Ce3+: 360 - 370nm
Gd3+ : 312 nm
Tb3+ : 480, 545nm
Dy3+ : 478, 500, 572, 585nm
Eu3+ : 610 nm
Sm3+ : 560, 602, 645nm
[CO]n*? intr. : 410nm
ST: 475nm
530 (?)
No spectrum yet
Comments on spectrum and activators:
The following emission centers have been found by steady-state spectroscopy: two different Mn2+ centers substituting for Ca2+ and Mg2+ and rare-earth elements such as Ce3+, Gd3+, Tb3+, Dy3+ and Sm3+ substituting for Ca2+ (Gorobets and Rogojine). Cathodoluminescence: Deconvolution of corrected CL spectra from dolomite shows two overlapping peaks at 17315 cm−1 (578 nm) and 15270 cm−1 (655 nm). In rare cases only the 655 nm Gaussian peak is present. The CL peak at 578nm (Ia) is due to Mn2+ in the Ca site and the peak at 655nm (Ib) to Mn2+ in the Mg position. The Mn2+ content in dolomite is linearly correlated with the normalized EPR areas of the two sites. The distribution ratio (KD) of Mn2+ in the two sites of dolomite, i.e. the Ca and the Mg position, was determined by EPR and CL spectroscopy. A linear correlation exists between KD from EPR and the intensity ratio IbIa from CL. CL spectroscopy is thus an appropriate method to determine KD in dolomite and evaporitic dolomites may be differentiated from non-evaporitic dolomites by their KD-values. (see: Mn2+-activated luminescence in dolomite, calcite and magnesite: quantitative determination of manganese and site distribution by EPR and CL spectroscopy, Ahmad El Ali et Al., Chemical Geology, Volume 104, Issues 1–4, 10 February 1993, Pages 189-202)
Best localities for fluorescence (*):
- Horn Silver Mine, Beaver County, Utah, USA;
- Langban, Varmland Province, Sweden (red SW);
- San Bernardo Quarry, Zandobbio, Bergamo Province, Lombardy, Italy (pink);
- Tsumeb Mine, Tsumeb, Oshikoto Region, Namibia (fluo orange);
(*)The data are not exhaustive and are limited to a few remarkable localities for fluorescence
Bibliographic reference for luminescence:
- The Henkel Glossary of Fluorescent Minerals, Dr. Gerhard Henkel, Published by the FMS, 1989 ,
- Luminescent Spectra of Minerals, Boris S. Gorobets and Alexandre A. Rogojine, Moscow, 2002 ,
Reference for luminescence on the Internet:
Images:
- Arraia-Maeztu, Álava, Basque Country, Spain: https://www.mindat.org/photo-556003.html
- Franklin, USA: https://www.mindat.org/photo-629957.html
Mineralogical reference on the Internet:
 http://www.mindat.org/show.php?name=Dolomite
http://www.mindat.org/show.php?name=Dolomite
 http://webmineral.com/data/Dolomite.shtml
http://webmineral.com/data/Dolomite.shtml
Internet Search:
 Image search on 'Google Images'
Image search on 'Google Images'
 Search for documents in all languages on Google
Search for documents in all languages on Google
A request providing no result means only that no such reference exists in the database, but it does not mean that what you are looking for does not exist, just not to our knowledge. If you think you have found an error or omission, please let us know via the contact page being sure to cite the source of information.