Database of luminescent minerals
TITANITE
Chemical formula: CaTiSiO5
Family: Silicates
Status: IMA-GP
Crystal system : Monoclinic
Display mineral: NON
Associated names (luminescent varieties, discredited names, synonyms, etc.): sphene,
Luminescence:
Shortwave UV (254nm) colors: |
Dark Orange /Tawn , | ||
Do you have a photo of this mineral you would like to see in the gallery? Contact us!
Phosphorescence (in the common sense of the term) observable with the naked eye:
No data
Activator(s) and spectrum:
Activator(s): Centres dûs aux effets des radiations, Cr3+, Ti3+, Sm3+, Eu3+, Er3+, Pr3+, Nd3+, Tm3+,
Peaks in the spectrum (nm):
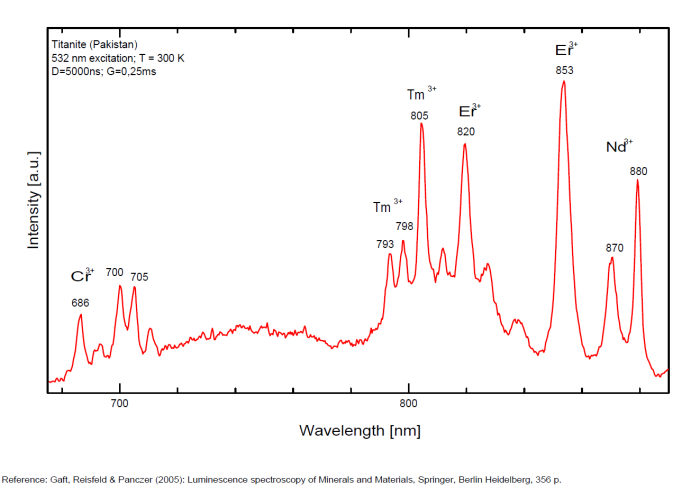
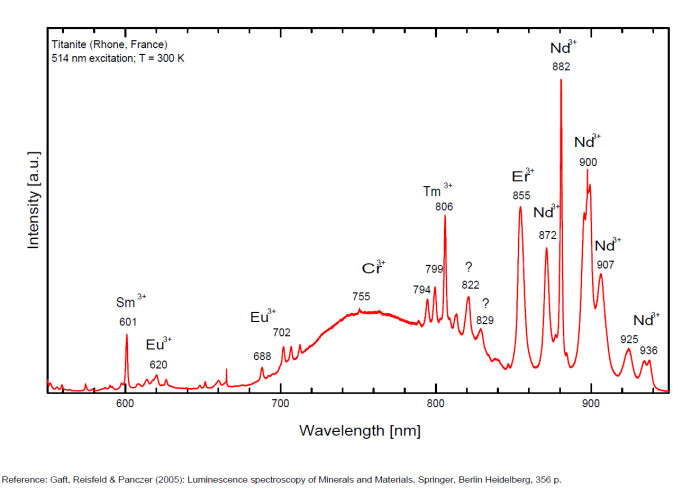
Sm3+ : 600nm Eu 3+ : 620, 703nm Ti3+? : 742 749 762 769 793 798 804 820nm Cr3+ : 750, 750, 786nm
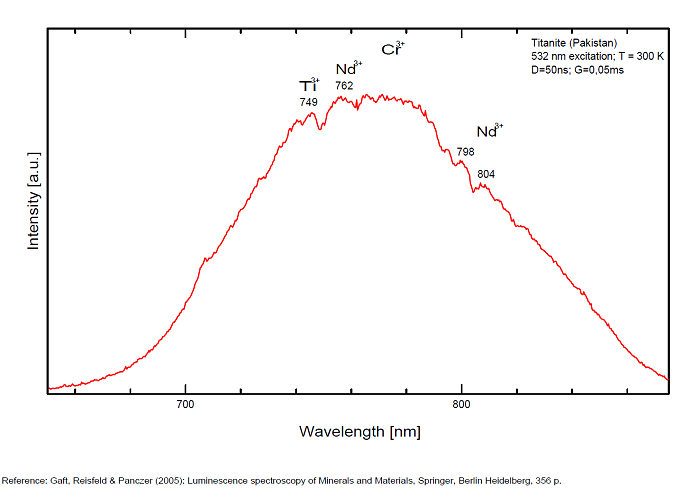
Spectrum: Michael Gaft, Petah Tikva, Israel. Plot: Institute of Mineralogy, University of Vienna, Austria, with permission of the authors.
Spectrum Galery:
 ...
...Comments on spectrum and activators:
Potential luminescent centers include intrinsic TiO6 and Ti3+ and different impurities such as trivalent and divalent rare-earth elements (REE), Pb2+ and Mn2+ substituting for Ca2+, Cr3+ and Mn4+ substituting for Ti4+, and Cr4+, Cr5+ and Fe3+ substituting for Si4+. Besides that, titanite can incorporate minor amounts of radioactive impurity components (particularly U and Th) that affect the crystal structure through α- and β-decay events. Thus radiation-induced luminescence centers are also possible. Nevertheless, steady-state luminescent spectroscopy of titanite under UV and X-ray excitations did not reveal characteristic bands and lines (Gorobets and Rogojine 2001) and only ionoluminescence spectrum of titanite exhibits various narrow lines of Sm3+, Eu3+ and Nd3+ (Yang 1995). Chromium and other metals, such as Nb, Ta, V, Mn, Mg, Sn, Al, and Fe, are generally considered to be incorporated at the six-fold coordinated Ti-site whereas REEs substitute Ca on its large, seven-coordinated site. (Gaft)
Best localities for fluorescence (*):
- Franklin Marble, Rudeville, New Jersey, USA (weak brown SW);
- Capelinha, Minas Gerais, Brazil;
- Kola Peninsula, Russia (dim brown SW);
(*)The data are not exhaustive and are limited to a few remarkable localities for fluorescence
Bibliographic reference for luminescence:
- The Henkel Glossary of Fluorescent Minerals, Dr. Gerhard Henkel, Published by the FMS, 1989 ,
- Fluorescence: Gems and Minerals Under Ultraviolet Light, Manuel Robbins, 1994, Geoscience Press, ISBN 0-945005-13-X ,
- Luminescence Spectroscopy of Minerals and Materials, M. Gaft, R. Reisfeld, G. Panczer, Springer Editor, ISBN: 10 3-540-21918-8 ,
Reference for luminescence on the Internet:
Mineralogical reference on the Internet:
 http://www.mindat.org/show.php?name=Titanite
http://www.mindat.org/show.php?name=Titanite
 http://webmineral.com/data/Titanite.shtml
http://webmineral.com/data/Titanite.shtml
Internet Search:
 Image search on 'Google Images'
Image search on 'Google Images'
 Search for documents in all languages on Google
Search for documents in all languages on Google
A request providing no result means only that no such reference exists in the database, but it does not mean that what you are looking for does not exist, just not to our knowledge. If you think you have found an error or omission, please let us know via the contact page being sure to cite the source of information.