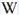Database of luminescent minerals
emerald (French name: emeraude )
Chemical formula: See BERYL
Family: Silicates
Status: NR
Crystal system : Hexagonal
Display mineral: NON
Luminescence:
Longwave UV (365nm) colors: |
Red , | ||
Daylight picture

Emeraude, Muzo, Colombia;
Photo and Copyright: Richard Loyens
Longwave (365nm) picture

Emeraude, Muzo, Colombia;
fluo red, excitation: laser 405nm
Photo and Copyright: Richard Loyens
Do you have a photo of this mineral you would like to see in the gallery? Contact us!
Phosphorescence (in the common sense of the term) observable with the naked eye:
No phosphorescence visible to the naked eye under any type of UV
Comments:
The gem variety of BERYL of green color.
Activator(s) and spectrum:
Activator(s): Cr3+, Fe3+, Mn4+, Mn2+ , V2+,
Peaks in the spectrum (nm):
Cr3+ : Lines at 682, 683, 684nm, Cr3+ replacing Al3+ : broad band peaking at 730nm Cr3+ : (666), 685, (696), Cr3+ I: 725nm (Band) Cr3+ II: 715nm (Band) Fe3+ : broad band at 735nm Cr3+ , V2+, Mn4+ : broad band at 720 (Lifetime = 100 microsecondes) VO4 : 423 (Lifetime = 1 microseconde)

Col. G. Barmarin; Spectre: G. Barmarin
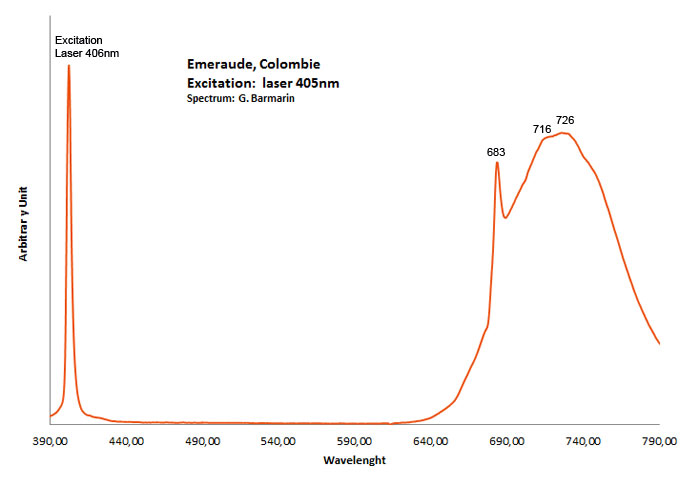
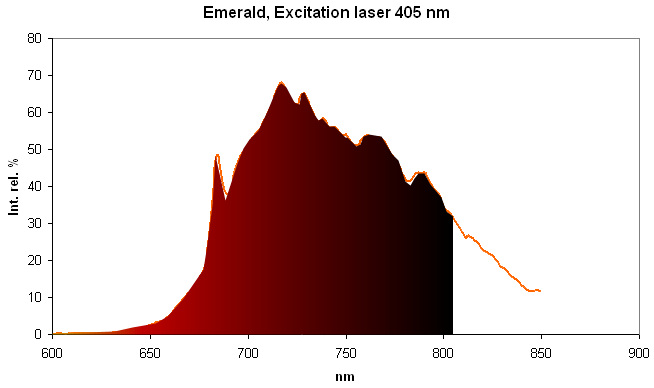
Spectrum Galery:

 ...
...Comments on spectrum and activators:
Steady-state emission of beryl was previously studied. The broad band at 720 nm is connected with Fe3+, while the relatively narrow bands at 480 and 570 nm are ascribed to Mn2+ in tetrahedral and octahedral coordination, correspondingly. Cr3+ emission was connected with narrow R-lines at 680 and 682 nm (Tarashchan 1978; Kuznetsov and Tarashchan 1988). (Cr3+ impurity ions in highly distorted octahedron sites) The Cr3+ luminescence properties in natural beryl minerals have been studied as a function of the Cr content as well as impurities such as Fe and V. It appears that the Cr3+ crystal field is linked to the Cr amount and decreases when Cr increases. A competition between Cr and V was noticed for very low Cr concentration (Ollier et al. 2015). (Gaft)
Best localities for fluorescence (*):
- Muzo Mine, Mun. de Muzo, Vasquez-Yacopí Mining District, Boyacá Department, Colombia;
- Brumado, Bahia, Brazil (red LW);
(*)The data are not exhaustive and are limited to a few remarkable localities for fluorescence
Bibliographic reference for luminescence:
- The Henkel Glossary of Fluorescent Minerals, Dr. Gerhard Henkel, Published by the FMS, 1989 ,
- Luminescence Spectroscopy of Minerals and Materials, M. Gaft, R. Reisfeld, G. Panczer, Springer Editor, ISBN: 10 3-540-21918-8 ,
- Luminescent Spectra of Minerals, Boris S. Gorobets and Alexandre A. Rogojine, Moscow, 2002 ,
Mineralogical reference on the Internet:
 http://www.mindat.org/show.php?name=Emerald
http://www.mindat.org/show.php?name=Emerald
 http://webmineral.com/data/Emerald.shtml
http://webmineral.com/data/Emerald.shtml
Internet Search:
 Image search on 'Google Images'
Image search on 'Google Images'
 Search for documents in all languages on Google
Search for documents in all languages on Google
A request providing no result means only that no such reference exists in the database, but it does not mean that what you are looking for does not exist, just not to our knowledge. If you think you have found an error or omission, please let us know via the contact page being sure to cite the source of information.