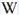Database of luminescent minerals
feldspar (French name: feldspath )
Chemical formula: See ALBITE /Group of minerals: ALBITE, MICROCLINE, SANIDINE
Family: Silicates
Status:
Display mineral: NON
Luminescence:
Shortwave UV (254nm) colors: |
Red , Blue , | ||
Do you have a photo of this mineral you would like to see in the gallery? Contact us!
Phosphorescence (in the common sense of the term) observable with the naked eye:
No data
Triboluminescence: OUI
Thermoluminescence: OUI
Comments:
In Iron-rich feldspars, luminescence emissions at longer wavelengths, i.e, 560 nm & 750 nm stem from point defects of Mn2+ and Fe3+ in feldspar lattices. Furthermore, high amounts of these impurities (e.g. 0.3%) lower these light emissions. Dark and iron-rich samples emit weak luminescence signals.
Activator(s) and spectrum:
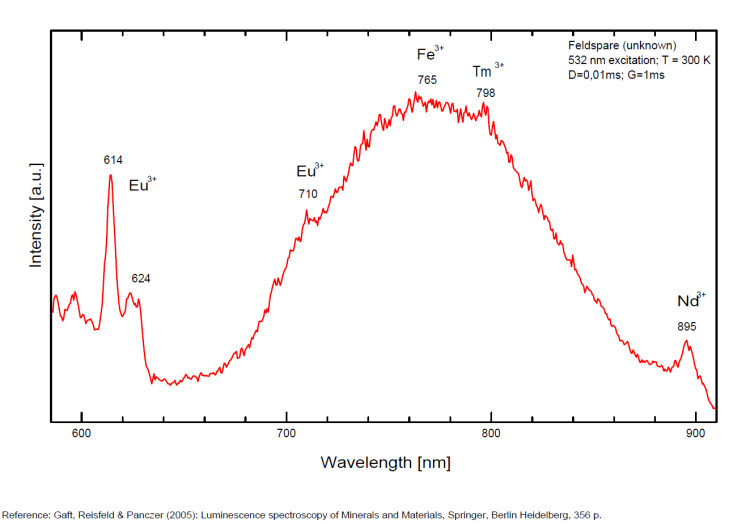
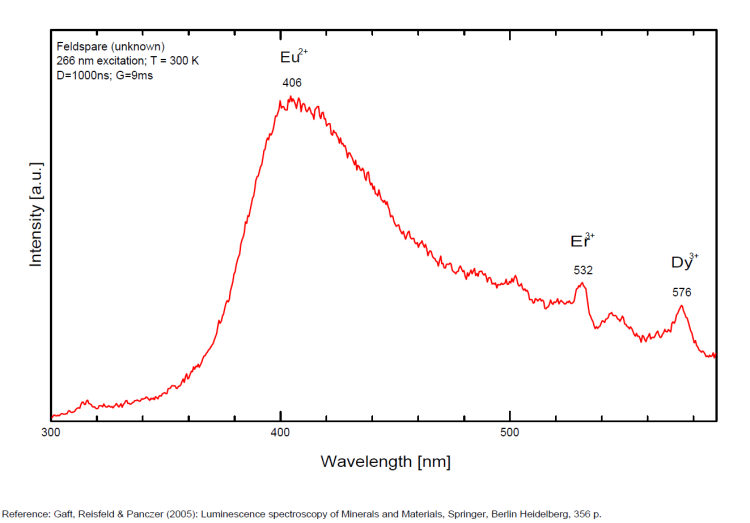
Activator(s): Fe3+, Pb2+, Eu2+, Ce3+, Sm3+, Dy3+, Tb3+, Mn2+ , Nd3+, Tm3+, Tl+,
Peaks in the spectrum (nm):
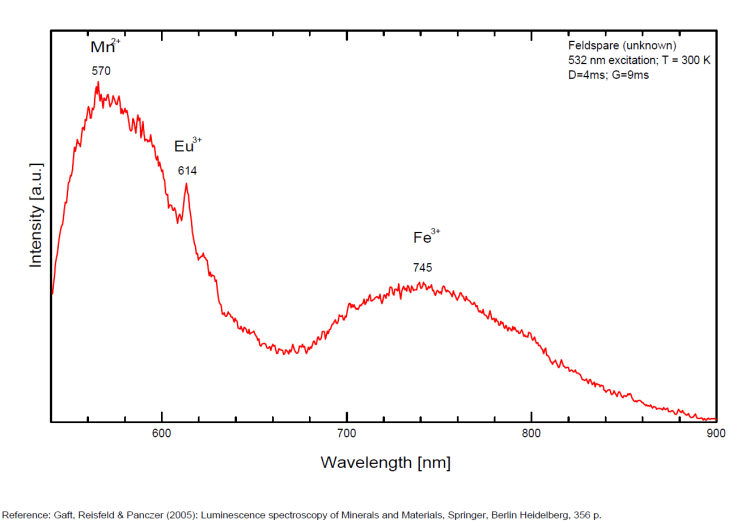
Eu3+ : 614, 624, 710nm
Fe3+ : large band at 735 - 741 to 765nm (FWHM: 120nm)
Tm3+ : 798nm
Nd3+ : 895nm
Pb+ : band at 851nm (FWHM : 50nm )
Spectrum: Michael Gaft, Petah Tikva, Israel. Plot: Institute of Mineralogy, University of Vienna, Austria, with permission of the authors.
Spectrum Galery:

 ...
...Comments on spectrum and activators:
Steady-state luminescence of feldspars is well studied. The following impurity centers have been found: Tl+ , Pb+, Pb2+, REE3+ (Ce, Dy, Sm, Tb, Nd), Eu2+, Mn2+, Fe3+, Cr3+ (Gaft) Luminescence of monovalent Pb was firstly proposed for explanation of IR luminescence band peaking at 860 nm in emission spectrum of feldspars The UV emission of feldspar at 290 nm is linked with the presence of Na and cracks-strain exsolution-twinning; at 340 nm with stress in Si—O bonds; the broad blue band (380-450nm) with [AlO4]o defects; at 560nm with Mn2+ point defects and at 720nm with iron in the lattice. Iron-rich feldspars: luminescence emissions at 560 nm and 750 nm come from point defects of Mn2+ and Fe3+ in feldspar lattices. Furthermore, high amounts of these impurities (e.g. 0.3%) lower these light emissions. Dark and iron-rich samples emit weak luminescence signals.
Best localities for fluorescence (*):
- Mount Malosa¸ Zombat District¸ Malawi;
(*)The data are not exhaustive and are limited to a few remarkable localities for fluorescence
Bibliographic reference for luminescence:
- The Henkel Glossary of Fluorescent Minerals, Dr. Gerhard Henkel, Published by the FMS, 1989 ,
- Fluorescence: Gems and Minerals Under Ultraviolet Light, Manuel Robbins, 1994, Geoscience Press, ISBN 0-945005-13-X ,
- The World of Fluorescent Minerals, Stuart Schneider, Schiffer Publishing, 2006, ISBN 0-7643-2544-2 ,
- Luminescence Spectroscopy of Minerals and Materials, M. Gaft, R. Reisfeld, G. Panczer, Springer Editor, ISBN: 10 3-540-21918-8 ,
Reference for luminescence on the Internet:
Mineralogical reference on the Internet:
 http://www.mindat.org/show.php?name=Feldspar
http://www.mindat.org/show.php?name=Feldspar
 http://webmineral.com/data/Feldspar.shtml
http://webmineral.com/data/Feldspar.shtml
Internet Search:
 Image search on 'Google Images'
Image search on 'Google Images'
 Search for documents in all languages on Google
Search for documents in all languages on Google
A request providing no result means only that no such reference exists in the database, but it does not mean that what you are looking for does not exist, just not to our knowledge. If you think you have found an error or omission, please let us know via the contact page being sure to cite the source of information.