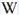Database of luminescent minerals
hackmanite
Chemical formula: See SODALITE
Family: Silicates
Status: NON APPR
Crystal system : Isometric
Display mineral: OUI
Luminescence:
Longwave UV (365nm) colors: |
Orange , Orangy yellow , Orange Red , | ||
Intensity LW:Strong | Frequency LW:Always | ||
Midwave UV (320nm) colors: |
Orange , Orangy yellow , | ||
Shortwave UV (254nm) colors: |
Orangy yellow , Bluish White , Yellowish White , Orangy yellow , Orange , Yellowish , | ||
Intensity SW:Medium | Frequency SW:Very often | ||
Daylight picture

Longwave (365nm) picture

hackmanite under UVLW,
Photo and Copyright: James Hamblen
Site of the author
Used with permission of the author
Pictures Galery:


 ...
...  Go to the galery (24 pictures)
Go to the galery (24 pictures)
Do you have a photo of this mineral you would like to see in the gallery? Contact us!
Phosphorescence (in the common sense of the term) observable with the naked eye:
Type d'UV |
Couleur |
Intensité |
Fréquence d'observation |
|---|---|---|---|
UV longs (365nm): | Bluish White | Medium | UV moyens (320 nm): | Bluish White | Very Strong | UV courts (254 nm): | Bluish White | Very Strong |
Tenebrescence: OUI

hackmanite, up after and down before exposition to SW (tenebrescence);
Koksha Valley, Badakhshan Province, Afghanistan;
;
Col. G.Barmarin; Photo: G. Barmarin
Thermoluminescence: OUI
Comments:
A sulfide rich sodalite and should not be regarded as a separate species.
Activator(s) and spectrum:
Activator(s): S2-,
Peaks in the spectrum (nm):
S2- : (566),(610), 625, 647, 664, (695), (723), (751nm)
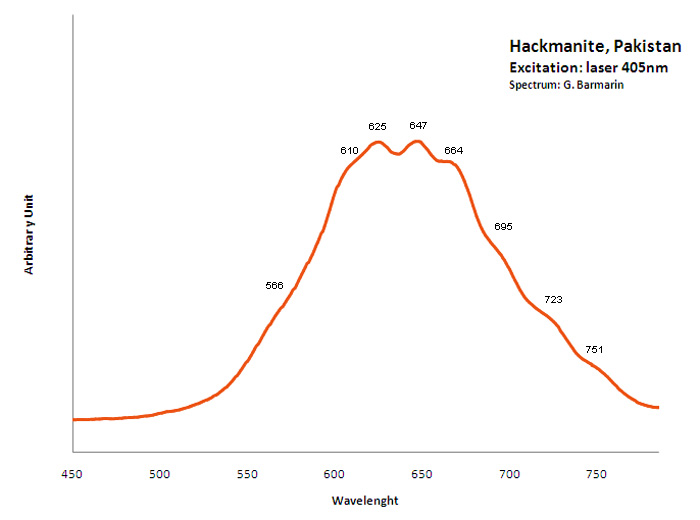
Col. G. Barmarin; Spectre: G. Barmarin
Spectrum Galery:

 ...
...Comments on spectrum and activators:
O. Ivan Lee investigated what he calls the reversible photosensitivity of hackmanite from Bancroft (Ontario) and his response to different UV sources as early as 1936. He presented the phenomenon for the 50th Anniversary Celebration Banquet of the New York Mineralogical Club, in November 18, 1936 at the American Museum of Natural History. It seems that it was the first observation and the first public announcement and publication (American Mineralogist vol 21) about photochromism (tenebrescence) in mineralogy. Chemical analyses revealed that the mineral contains a certain amount of sulfur as a substitute for chlorine in the crystal structure. The FTIR spectra of hackmanite showed that the samples contain water. The stretching vibration peak of water of crystallization (H2O) occurs at 3438 cm-1 and the bending peak is at 1623 cm-1. Its tenebrescence is caused by hole color centers which are contributed to the presence of sulfur (S2-)) and to some negatively charged chlorine atoms being missing in the crystal structure of hackmanite. (source: http://www.geology.com.cn/Geology-Journals/article-35765.html) Crystals of Hackmanite of Koksha Valley in Afghanistan are usually found in a matrix constituted by non-fluorescing Winchite and/or marble. Synthetic sodalites containing sulfur and showing considerable photochromic activity have been investigated by ESR. The center responsible for the color has been shown to be an electron trapped at a chlorine vacancy. The origin of the electron which is reversibly transferred during the processes of coloration and bleaching is believed to be the ion S2-). (see William G. Hodgson, Jacob S. Brinen, and Emil F. Williams, Electron Spin Resonance Investigation of Photochromic Sodalites, The Journal of Chemical Physics 47, 3719 , 1967)
Best localities for fluorescence (*):
- The Ilímaussaq Complex, Greenland;
- Svintsovyi Ruchei (Lead Creek), Kukisvumchorr Mt, Khibiny Massif, Kola Peninsula, Murmanskaja Oblast, Northern Region, Russia;
- Tawa valley, Kola Peninsula, Murmanskaja Oblast, Northern Region, Russia (type locality);
- Davis Quarry, Dungannon Township, Bancroft District, Hastings Co., Ontario, Canada;
- Poudrette quarry (or Demix or Uni-Mix or Desourdy quarry), Mont Saint-Hilaire, La Vallée-du-Richelieu RCM, Montérégie, Québec, Canada;
- Princess Sodalite quarry and Cancrinite Hill, Bancroft, Canada;
- Ladjuar Medam, Sar e Sang, Koksha Valley, Khash & Kuran Wa Munjan Districts, Badakhshan Province, Afghanistan;
- Kiran, Koksha Valley, Khash & Kuran Wa Munjan Districts, Badakhshan Province, Afghanistan
- Pein-Pyit, Mogok Township, Pyin-Oo-Lwin District, Mandalay Division, Burma (fluo+strong tenebrescence);
- Saga I Quarry Morje Porsgrunn, Sagasen Quarry and Ostskogen Quarry in Tvedalen, Langesundfjord, Larvik Plutonic Complex, Norway;
- Cancrinite Hill, Dungannon Township, Hastings Co., Ontario, Canada (dark blue sodalite dull red fluorescing with yellow cancrinite);
- Tashmarine (diopside) mine, Tien Shan Mountains, Xinjiang Uyghur Autonomous Region, China;
(*)The data are not exhaustive and are limited to a few remarkable localities for fluorescence
Bibliographic reference for luminescence:
- The Henkel Glossary of Fluorescent Minerals, Dr. Gerhard Henkel, Published by the FMS, 1989 ,
- Fluorescence: Gems and Minerals Under Ultraviolet Light, Manuel Robbins, 1994, Geoscience Press, ISBN 0-945005-13-X ,
- The World of Fluorescent Minerals, Stuart Schneider, Schiffer Publishing, 2006, ISBN 0-7643-2544-2 ,
- Ultraviolet Light and Fluorescent Minerals, Th. Warren, S. Gleason, R. Bostwick, et E. Verbeek, 1995, ISBN 0-9635098-0-2 ,
- Minershop.com ,
- Luminescenza nel regno minerale, Guido Mazzoleni, fotografia Roberto Appiani, Libri Sandit, 2010, ISBN 978-88-95990-63-7 ,
- Handbook of Fluorescent Gems and Minerals, a practical guide for the gem and mineral collector, Jack de Ment, 1949 ,
Reference for luminescence on the Internet:
- Hackmanite as a gemstone
- Poudrette Quarry, Mont St Hilaire, Canada (Mindat.org)
- The Langesundsfjord: history, geology, pegmatites, minerals, Alf Olav Larsen, Bode Verlag Gmbh, 2010 ISBN 978-3-925094-97-2
- Video "Tenebrescence of a Hackmanite cabochon"
- http://rruff.info/uploads/CM21_549.pdf
- A New Property of Matter: Reversible Photosensitivity in Hackmanite from Bancroft, Ontario, O. Ivan Lee, Am. Min, vol 21, 1936
- http://www.geology.com.cn/Geology-Journals/article-35765.html
- https://www.academia.edu/21548029/Laser-induced_time-resolved_luminescence_of_tugtupite_sodalite_and_hackmanite
- Fotochromisme en fluorescentie in sodaliet, variëteit hackmaniet, Geonieuws (MKA) Juni 2010
- MONT-SAINT-HILAIRE, History, Geology, Mineralogy, Laszlo HORVATH, The Canadian Mineralogist, Special Publication 14, 2019
- Photochromism, UV-Vis, Vibrational and Fluorescence Spectroscopy of Differently Colored Hackmanite, November 2023, Crystals 13:1607 DOI:10.3390/cryst13111607
Images:
- Koksha Valley, Afghanistan: http://www.mindat.org/photo-345828.html
- Ontario, Canada: http://www.mindat.org/photo-75890.html
Video:
- Ténébrescence hackmanite & tugtupite : https://www.youtube.com/watch?v=stDh8GDkEYc
- Ténébrescence Hackmanite Mogok : https://www.youtube.com/watch?v=k9B7Omm0nmk
- Ténébrescence hackmanite : https://www.instagram.com/p/BMAb0UWD0nn
Mineralogical reference on the Internet:
 http://www.mindat.org/show.php?name=Hackmanite
http://www.mindat.org/show.php?name=Hackmanite
 http://webmineral.com/data/Hackmanite.shtml
http://webmineral.com/data/Hackmanite.shtml
Internet Search:
 Image search on 'Google Images'
Image search on 'Google Images'
 Search for documents in all languages on Google
Search for documents in all languages on Google
A request providing no result means only that no such reference exists in the database, but it does not mean that what you are looking for does not exist, just not to our knowledge. If you think you have found an error or omission, please let us know via the contact page being sure to cite the source of information.