Database of luminescent minerals
CLINOHUMITE
Chemical formula: (Mg,Fe+2)9(SiO4)4(F,OH)2
Family: Silicates
Status: IMA-GP
Crystal system : Monoclinic
Display mineral: NON
Luminescence:
Shortwave UV (254nm) colors: |
Orange , Orangy yellow , | ||
Intensity SW:Strong | |||
Daylight picture

CLINOHUMITE, Tajikistan;
Col. G.Barmarin; Photo: G. Barmarin
Shortwave (254nm) picture

CLINOHUMITE, Tajikistan; UVSW
Col. G.Barmarin; Photo: G. Barmarin
Do you have a photo of this mineral you would like to see in the gallery? Contact us!
Phosphorescence (in the common sense of the term) observable with the naked eye:
No data
Activator(s) and spectrum:
Activator(s): Mn2+ , Fe3+, TiO6, n[TiO6] cluster,
Peaks in the spectrum (nm):
TiOn (Ti4+ - Si4+) : 500nm n[TiOn] : 550-600nm Mn2+ repl. Mg2+ : 630 - 650nm Fe3+ : broad band at 745nm OH (?) : 660nm (peak)
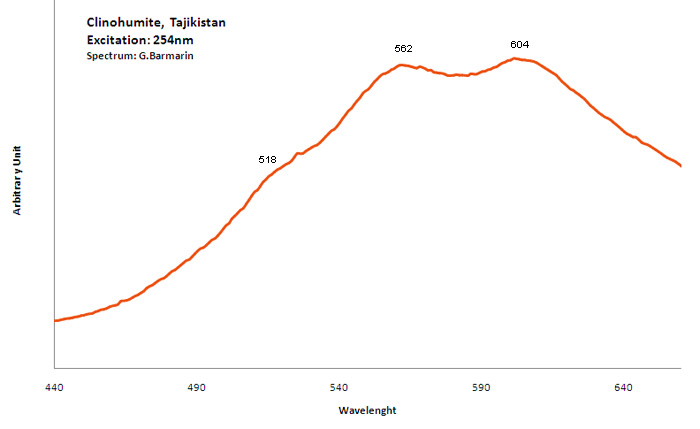
Col. G. Barmarin; Spectre: G. Barmarin
Spectrum Galery:
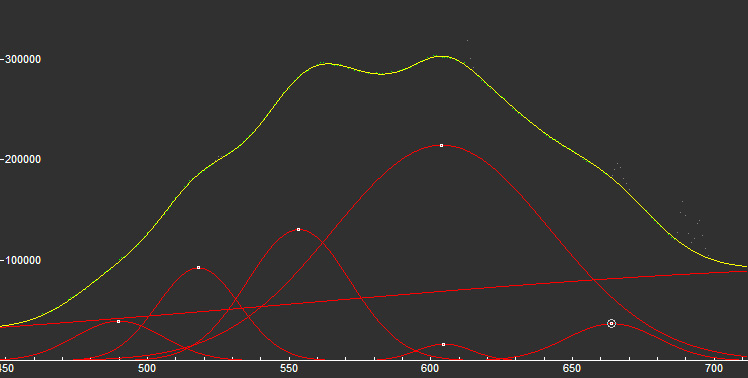
 ...
...Comments on spectrum and activators:
Steady state luminescence of clinohumite was connected to Mn2+ and possible TiO6 centers (Gorobets and Rogojine 2001). Excitation by CW laser with 532 nm revealed two emission bands which may be ascribed to Mn2+ and Fe3+ (Gaft)
Best localities for fluorescence (*):
- Pamir Mountains, Tadzhikistan;
- Lake Albano (south of Rome), Italy;
(*)The data are not exhaustive and are limited to a few remarkable localities for fluorescence
Bibliographic reference for luminescence:
- Luminescence Spectroscopy of Minerals and Materials, M. Gaft, R. Reisfeld, G. Panczer, Springer Editor, ISBN: 10 3-540-21918-8 ,
- Luminescent Spectra of Minerals, Boris S. Gorobets and Alexandre A. Rogojine, Moscow, 2002 ,
Mineralogical reference on the Internet:
 http://www.mindat.org/show.php?name=Clinohumite
http://www.mindat.org/show.php?name=Clinohumite
 http://webmineral.com/data/Clinohumite.shtml
http://webmineral.com/data/Clinohumite.shtml
Internet Search:
 Image search on 'Google Images'
Image search on 'Google Images'
 Search for documents in all languages on Google
Search for documents in all languages on Google
A request providing no result means only that no such reference exists in the database, but it does not mean that what you are looking for does not exist, just not to our knowledge. If you think you have found an error or omission, please let us know via the contact page being sure to cite the source of information.